Oxygen's Versatility in Bond Formation

Oxygen is a highly reactive element, and its ability to form bonds with other elements is crucial for many biological and chemical processes. In this article, we will explore the various types of bonds that oxygen can form with other elements and discuss the factors that influence its bonding capabilities.
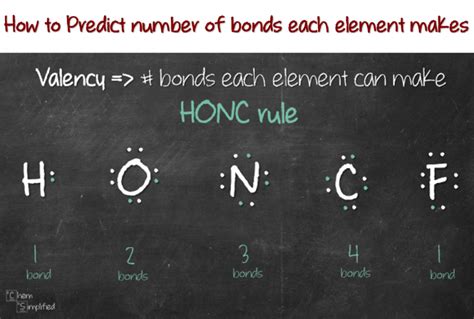
Oxygen's atomic structure plays a significant role in its ability to form bonds. With six valence electrons, oxygen is highly electronegative, which means it has a strong tendency to attract electrons towards itself. This property allows oxygen to form a wide range of bonds with other elements, from strong covalent bonds to weaker ionic bonds.
Covalent Bonds
Oxygen can form covalent bonds with many elements, including hydrogen, carbon, nitrogen, and sulfur. Covalent bonds involve the sharing of electrons between atoms, and oxygen's high electronegativity makes it an excellent partner for sharing electrons.
One of the most common covalent bonds formed by oxygen is the hydroxyl (-OH) bond, which is a crucial component of many biomolecules, including water, sugars, and amino acids. Oxygen can also form covalent bonds with carbon, resulting in the formation of carbon-oxygen double bonds, which are a key feature of many organic compounds.
Double and Triple Bonds
Oxygen's ability to form double and triple bonds with other elements is also significant. Double bonds involve the sharing of two pairs of electrons, while triple bonds involve the sharing of three pairs of electrons. Oxygen's high electronegativity makes it an excellent partner for forming double and triple bonds.
For example, oxygen can form a double bond with carbon, resulting in the formation of a carbonyl group (C=O), which is a key feature of many organic compounds, including sugars, amino acids, and fatty acids. Oxygen can also form a triple bond with nitrogen, resulting in the formation of a nitro group (N=O), which is a key feature of many explosives and pharmaceuticals.
Ionic Bonds
In addition to covalent bonds, oxygen can also form ionic bonds with other elements. Ionic bonds involve the transfer of electrons between atoms, resulting in the formation of ions with opposite charges.
Oxygen's high electronegativity makes it an excellent partner for forming ionic bonds. For example, oxygen can form an ionic bond with sodium, resulting in the formation of sodium oxide (Na2O). Oxygen can also form an ionic bond with calcium, resulting in the formation of calcium oxide (CaO).
Factors Influencing Oxygen's Bonding Capabilities

Several factors influence oxygen's bonding capabilities, including its atomic structure, electronegativity, and the presence of other elements.
- Atomic structure: Oxygen's atomic structure, including its six valence electrons, plays a significant role in its ability to form bonds.
- Electronegativity: Oxygen's high electronegativity makes it an excellent partner for forming covalent bonds and ionic bonds.
- Presence of other elements: The presence of other elements, such as hydrogen, carbon, and nitrogen, can influence oxygen's bonding capabilities.
- Pressure and temperature: Changes in pressure and temperature can influence oxygen's bonding capabilities, particularly in the formation of ionic bonds.
Biological Significance of Oxygen's Bonding Capabilities
Oxygen's bonding capabilities play a crucial role in many biological processes, including:
- Respiration: Oxygen's ability to form bonds with other elements is essential for cellular respiration, which involves the breakdown of glucose to produce energy.
- Photosynthesis: Oxygen's ability to form bonds with other elements is also essential for photosynthesis, which involves the conversion of carbon dioxide and water into glucose and oxygen.
- Protein synthesis: Oxygen's ability to form bonds with other elements is essential for protein synthesis, which involves the formation of peptide bonds between amino acids.
Industrial Applications of Oxygen's Bonding Capabilities

Oxygen's bonding capabilities have many industrial applications, including:
- Steel production: Oxygen is used to produce steel, which involves the formation of iron oxide (Fe2O3) through the reaction of iron with oxygen.
- Pharmaceuticals: Oxygen's ability to form bonds with other elements is essential for the production of many pharmaceuticals, including antibiotics and painkillers.
- Fertilizers: Oxygen's ability to form bonds with other elements is also essential for the production of fertilizers, which involves the formation of ammonia (NH3) through the reaction of nitrogen with hydrogen.
Conclusion and Future Directions
In conclusion, oxygen's bonding capabilities play a crucial role in many biological and chemical processes. Understanding the factors that influence oxygen's bonding capabilities can provide valuable insights into its behavior and applications.
Future research directions may include:
- Exploring new bonding capabilities: Further research is needed to explore new bonding capabilities of oxygen, particularly in the context of emerging technologies such as quantum computing and nanotechnology.
- Developing new applications: New applications of oxygen's bonding capabilities may be developed in fields such as biotechnology, pharmaceuticals, and materials science.
- Improving industrial processes: Improving industrial processes that utilize oxygen's bonding capabilities can lead to more efficient and sustainable production of goods and services.
How many bonds can oxygen form with other elements?
+Oxygen can form multiple bonds with other elements, including covalent bonds, double bonds, and triple bonds. The exact number of bonds that oxygen can form depends on the specific element and the conditions under which the bond is formed.
What is the significance of oxygen's bonding capabilities in biology?
+Oxygen's bonding capabilities play a crucial role in many biological processes, including respiration, photosynthesis, and protein synthesis. Understanding oxygen's bonding capabilities is essential for understanding many biological processes and developing new treatments for diseases.
What are some industrial applications of oxygen's bonding capabilities?
+Oxygen's bonding capabilities have many industrial applications, including steel production, pharmaceuticals, and fertilizers. Understanding oxygen's bonding capabilities is essential for developing new technologies and improving industrial processes.