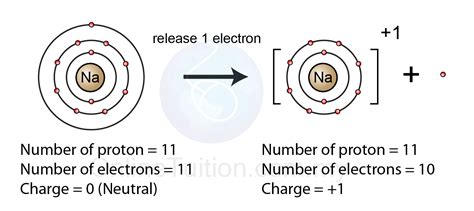
When it comes to forming ions, atoms tend to gain or lose electrons to achieve a stable electronic configuration, often mimicking the noble gas configuration. The formation of a 1+ ion, also known as a cation, involves the loss of one electron from a neutral atom. But which atom forms a 1+ ion most easily?
To answer this question, we need to consider the factors that influence the ease of ion formation. These factors include the atomic radius, electronegativity, and ionization energy of the atom.
Understanding Ionization Energy
Ionization energy is the minimum energy required to remove an electron from a neutral atom in its ground state. Atoms with low ionization energies tend to lose electrons more easily, making it simpler for them to form cations.

Atomic Radius and Ionization Energy
The atomic radius plays a significant role in determining the ionization energy of an atom. Atoms with larger atomic radii tend to have lower ionization energies, as the outermost electrons are farther away from the nucleus and experience a weaker attractive force.
Electronegativity and Ionization Energy
Electronegativity is a measure of an atom's ability to attract electrons in a covalent bond. Atoms with high electronegativity values tend to have higher ionization energies, as they hold onto their electrons more tightly.

Factors Influencing the Formation of 1+ Ions
Considering the factors mentioned above, the formation of a 1+ ion is most likely to occur in atoms with:
- Low ionization energies
- Large atomic radii
- Low electronegativity values
Identifying the Atom that Forms a 1+ Ion Most Easily
Based on the periodic trends and factors discussed above, the atom that forms a 1+ ion most easily is:
- Lithium (Li)
Lithium has a relatively low ionization energy of 520 kJ/mol, a large atomic radius of 152 pm, and a low electronegativity value of 0.98. These factors make it easy for lithium to lose one electron and form a 1+ ion.

Other atoms that tend to form 1+ ions easily include:
- Sodium (Na)
- Potassium (K)
- Rubidium (Rb)
- Caesium (Cs)
These atoms are all part of the alkali metal group and have similar properties that make it easy for them to lose one electron and form a 1+ ion.
Practical Applications of 1+ Ions
The formation of 1+ ions has several practical applications in various fields, including:
- Chemistry: 1+ ions are involved in many chemical reactions, such as acid-base reactions and redox reactions.
- Biology: 1+ ions play a crucial role in many biological processes, such as nerve impulses and muscle contractions.
- Materials Science: 1+ ions are used in the development of new materials, such as ion-conducting ceramics and energy storage devices.

Conclusion
In conclusion, the atom that forms a 1+ ion most easily is lithium, due to its low ionization energy, large atomic radius, and low electronegativity value. Understanding the factors that influence ion formation is crucial in many fields, from chemistry and biology to materials science.
We encourage you to share your thoughts and questions in the comments section below!
FAQ Section:
What is ionization energy?
+
Which atom has the lowest ionization energy?
+Cesium (Cs) has the lowest ionization energy, with a value of 375.7 kJ/mol.
What are some practical applications of 1+ ions?
+