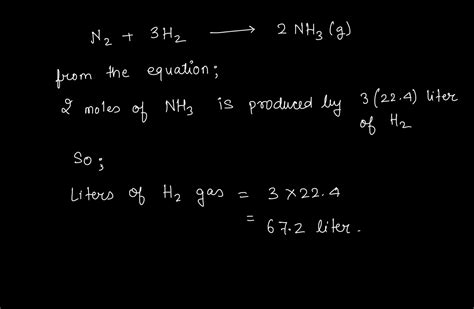Hydrogen and nitrogen are two of the most abundant elements in the universe, and their reaction to form ammonia is a crucial process that occurs naturally in various environments. Ammonia, a compound consisting of one nitrogen atom and three hydrogen atoms, plays a vital role in many biological and chemical processes. In this article, we will delve into the natural processes that lead to the formation of ammonia from hydrogen and nitrogen.

The Importance of Ammonia
Ammonia is a critical compound in many ecosystems, serving as a source of nitrogen for plants, microorganisms, and other organisms. Nitrogen is an essential nutrient for life, and ammonia is one of the primary forms in which it is available. The formation of ammonia from hydrogen and nitrogen is a vital process that supports the nitrogen cycle, which is essential for maintaining the balance of life on Earth.
Natural Processes of Ammonia Formation
There are several natural processes that lead to the formation of ammonia from hydrogen and nitrogen. Some of these processes occur in the atmosphere, while others take place in aquatic environments or within the Earth's crust.
Atmospheric Processes
In the atmosphere, ammonia is formed through the reaction of nitrogen and hydrogen gases. This reaction occurs in the presence of lightning, which provides the necessary energy to initiate the reaction. The reaction is as follows:
N2 + 3H2 → 2NH3
This process is known as the Haber-Bosch process, named after the German chemist Fritz Haber, who first described it in the early 20th century. Although this process is not as efficient as industrial methods, it is an essential natural process that contributes to the formation of ammonia in the atmosphere.
Aquatic Processes
In aquatic environments, ammonia is formed through the decomposition of organic matter. This process involves the breakdown of nitrogen-rich compounds, such as amino acids and nucleotides, by microorganisms. The decomposition process releases ammonia, which is then available for uptake by other organisms.
Geological Processes
In the Earth's crust, ammonia is formed through the interaction of nitrogen-rich minerals with hydrogen-rich fluids. This process occurs in areas where tectonic activity has created fractures and faults, allowing hydrogen-rich fluids to interact with nitrogen-rich minerals.
Biological Processes
In addition to these natural processes, ammonia is also formed through biological processes. Certain microorganisms, such as bacteria and archaea, have the ability to convert nitrogen gas into ammonia through a process known as nitrogen fixation. This process involves the reduction of nitrogen gas to ammonia, using energy derived from the breakdown of organic matter.
Nitrogen Fixation
Nitrogen fixation is a critical process that supports the nitrogen cycle, allowing nitrogen to be converted into a form that can be used by other organisms. This process is carried out by specialized microorganisms, such as rhizobia, which live in symbiosis with plants.

Environmental Factors Affecting Ammonia Formation
Several environmental factors can affect the formation of ammonia from hydrogen and nitrogen. These factors include temperature, pressure, and the presence of catalysts.
Temperature
Temperature plays a critical role in the formation of ammonia, as it affects the rate of the reaction. Higher temperatures can increase the rate of reaction, while lower temperatures can slow it down.
Pressure
Pressure also affects the formation of ammonia, as it can influence the equilibrium of the reaction. Higher pressures can favor the formation of ammonia, while lower pressures can favor the reverse reaction.
Catalysts
Catalysts can also affect the formation of ammonia, as they can lower the energy barrier required for the reaction to occur. Certain metals, such as iron and nickel, can act as catalysts for the Haber-Bosch process.

Conclusion
In conclusion, the reaction of hydrogen and nitrogen to form ammonia is a natural process that occurs in various environments. This process is essential for supporting the nitrogen cycle, which is critical for life on Earth. Understanding the factors that affect this process can provide valuable insights into the natural world and inform strategies for managing nitrogen resources.
What You Can Do
If you are interested in learning more about the natural processes that shape our world, we encourage you to explore further. You can start by reading about the nitrogen cycle and its importance for life on Earth. You can also explore ways to reduce your impact on the environment, such as reducing energy consumption and using sustainable practices.
FAQ
What is the Haber-Bosch process?
+The Haber-Bosch process is a natural process that occurs in the atmosphere, in which nitrogen and hydrogen gases react to form ammonia.
What is nitrogen fixation?
+Nitrogen fixation is a biological process that involves the conversion of nitrogen gas into ammonia, using energy derived from the breakdown of organic matter.
Why is ammonia important for life on Earth?
+Ammonia is an essential nutrient for life, serving as a source of nitrogen for plants, microorganisms, and other organisms.