Sulfur is a versatile element that can form a variety of compounds with other elements. One of the key aspects of sulfur's chemistry is its ability to form bonds with other atoms. But have you ever wondered how many bonds sulfur can form at once?
Sulfur's unique electron configuration allows it to form a range of bonds, from single to triple bonds, with other elements. In this article, we'll delve into the world of sulfur chemistry and explore the different types of bonds that sulfur can form, as well as the maximum number of bonds it can form at once.

Understanding Sulfur's Electron Configuration
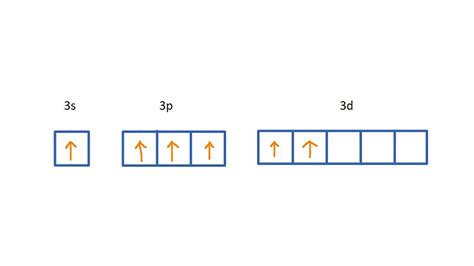
To understand how many bonds sulfur can form, we need to look at its electron configuration. Sulfur is a member of the chalcogen family, which includes elements like oxygen, selenium, and tellurium. Sulfur's electron configuration is [Ne] 3s² 3p⁴, which means it has six valence electrons in its outermost energy level.
Sulfur's electron configuration allows it to form a range of bonds, including single, double, and triple bonds. Single bonds involve the sharing of one pair of electrons between two atoms, while double and triple bonds involve the sharing of two and three pairs of electrons, respectively.
Types of Bonds Sulfur Can Form
Sulfur can form a variety of bonds with other elements, including:
- Single bonds: Sulfur can form single bonds with elements like hydrogen, carbon, and oxygen. These bonds involve the sharing of one pair of electrons between the sulfur atom and the other element.
- Double bonds: Sulfur can form double bonds with elements like oxygen and carbon. These bonds involve the sharing of two pairs of electrons between the sulfur atom and the other element.
- Triple bonds: Sulfur can form triple bonds with elements like nitrogen and carbon. These bonds involve the sharing of three pairs of electrons between the sulfur atom and the other element.

How Many Bonds Can Sulfur Form At Once?
So, how many bonds can sulfur form at once? The answer depends on the type of bond and the other elements involved.
- Single bonds: Sulfur can form a maximum of four single bonds at once. This is because sulfur has four unpaired electrons in its outermost energy level, which can be shared with other elements to form single bonds.
- Double bonds: Sulfur can form a maximum of two double bonds at once. This is because double bonds involve the sharing of two pairs of electrons, and sulfur has only two pairs of electrons available for bonding.
- Triple bonds: Sulfur can form a maximum of one triple bond at once. This is because triple bonds involve the sharing of three pairs of electrons, and sulfur has only one set of three electrons available for bonding.

Examples of Sulfur Compounds
Sulfur forms a wide range of compounds with other elements, including:
- Hydrogen sulfide (H₂S): This compound involves the formation of single bonds between sulfur and hydrogen.
- Sulfur dioxide (SO₂): This compound involves the formation of double bonds between sulfur and oxygen.
- Sulfur trioxide (SO₃): This compound involves the formation of double and single bonds between sulfur and oxygen.

Conclusion and Final Thoughts
In conclusion, sulfur is a versatile element that can form a range of bonds with other elements. The number of bonds sulfur can form at once depends on the type of bond and the other elements involved. Sulfur can form a maximum of four single bonds, two double bonds, and one triple bond at once.
We hope this article has provided you with a deeper understanding of sulfur's chemistry and its ability to form bonds with other elements. Whether you're a student of chemistry or simply interested in learning more about the elements, we hope you've found this article informative and engaging.

Now it's your turn! Share your thoughts and questions about sulfur's chemistry in the comments below. Do you have any favorite sulfur compounds or reactions? Let us know!
What is sulfur's electron configuration?
+Sulfur's electron configuration is [Ne] 3s² 3p⁴.
How many single bonds can sulfur form at once?
+Sulfur can form a maximum of four single bonds at once.
What is an example of a sulfur compound that involves the formation of triple bonds?
+An example of a sulfur compound that involves the formation of triple bonds is sulfur trioxide (SO₃).