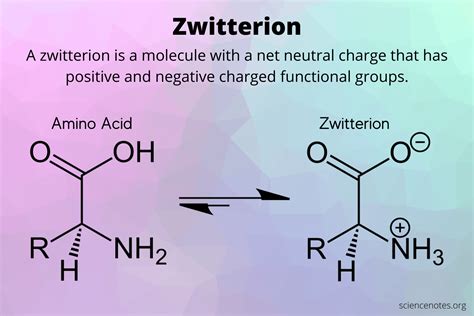
Amino acids are the building blocks of proteins, which are essential for various bodily functions, including growth, repair, and maintenance of tissues. One of the unique characteristics of amino acids is their ability to exist in different forms, including the zwitterionic form. In this article, we will delve into the world of zwitterionic amino acids, exploring their structure, properties, and significance in biological systems.
What are Zwitterionic Amino Acids?

Amino acids can exist in three main forms: cationic, anionic, and zwitterionic. The zwitterionic form is a unique state where the amino acid has both a positive and a negative charge, resulting in a net neutral charge. This occurs when the amino group (NH2) is protonated, and the carboxyl group (COOH) is deprotonated. The resulting zwitterion has a positive charge on the nitrogen atom and a negative charge on the oxygen atom.
Structure of Zwitterionic Amino Acids
The zwitterionic form of amino acids is characterized by the presence of both a positive and a negative charge. The general structure of a zwitterionic amino acid can be represented as:
NH3+ - CHR - COO-
where NH3+ is the protonated amino group, CHR is the side chain, and COO- is the deprotonated carboxyl group.
Properties of Zwitterionic Amino Acids

The zwitterionic form of amino acids has several unique properties that distinguish it from other forms:
- Solubility: Zwitterionic amino acids are highly soluble in water due to their ability to form hydrogen bonds with water molecules.
- Stability: The zwitterionic form is more stable than the cationic or anionic forms, as the positive and negative charges are balanced.
- Reactivity: Zwitterionic amino acids are less reactive than the cationic or anionic forms, as the positive and negative charges are neutralized.
Significance of Zwitterionic Amino Acids in Biological Systems
The zwitterionic form of amino acids plays a crucial role in various biological processes, including:
- Protein structure and function: Zwitterionic amino acids are essential for the stability and function of proteins, as they participate in hydrogen bonding and ionic interactions.
- Cell membrane transport: Zwitterionic amino acids can cross cell membranes more easily than the cationic or anionic forms, facilitating the transport of amino acids into cells.
- Enzyme activity: Zwitterionic amino acids can act as substrates or cofactors for enzymes, influencing enzyme activity and specificity.
Factors Affecting the Zwitterionic Form of Amino Acids

Several factors can influence the zwitterionic form of amino acids, including:
- pH: The pH of the solution can affect the zwitterionic form of amino acids, as changes in pH can alter the protonation state of the amino and carboxyl groups.
- Temperature: Temperature can influence the stability and reactivity of zwitterionic amino acids, as higher temperatures can increase the energy of the molecules.
- Concentration: The concentration of amino acids can affect the zwitterionic form, as high concentrations can lead to the formation of aggregates or crystals.
Applications of Zwitterionic Amino Acids
The zwitterionic form of amino acids has various applications in fields such as:
- Pharmaceuticals: Zwitterionic amino acids can be used as excipients or active ingredients in pharmaceutical formulations, exploiting their unique properties.
- Biotechnology: Zwitterionic amino acids can be used as substrates or cofactors for enzymes, influencing enzyme activity and specificity.
- Food industry: Zwitterionic amino acids can be used as food additives or supplements, enhancing the nutritional value and stability of food products.
Conclusion
In conclusion, the zwitterionic form of amino acids is a unique and essential state that plays a crucial role in various biological processes. Understanding the structure, properties, and significance of zwitterionic amino acids can provide insights into their applications in fields such as pharmaceuticals, biotechnology, and the food industry.
What is the zwitterionic form of amino acids?
+The zwitterionic form of amino acids is a state where the amino acid has both a positive and a negative charge, resulting in a net neutral charge.
What are the properties of zwitterionic amino acids?
+Zwitterionic amino acids have unique properties, including high solubility, stability, and reactivity.
What are the applications of zwitterionic amino acids?
+Zwitterionic amino acids have various applications in fields such as pharmaceuticals, biotechnology, and the food industry.