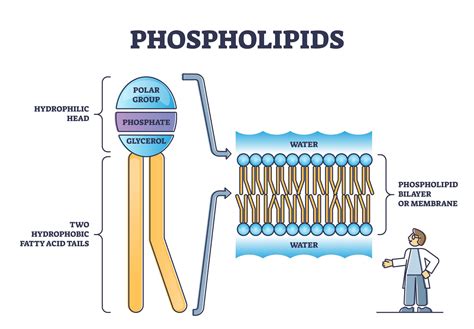
The phospholipid bilayer is a fundamental component of cell membranes, providing a selectively permeable barrier that regulates the movement of substances in and out of cells. The unique structure of the phospholipid bilayer, with its hydrophilic heads and hydrophobic tails, is crucial for its function. But why do phospholipid bilayers form the way they do? Here are five reasons:
The importance of understanding phospholipid bilayers cannot be overstated. As the primary structural component of cell membranes, they play a critical role in maintaining cellular homeostasis, regulating cell signaling, and facilitating the transport of molecules across the membrane. Yet, despite their significance, the phospholipid bilayer remains a complex and fascinating structure that continues to be the subject of scientific investigation.
One of the primary reasons phospholipid bilayers form the way they do is due to the amphipathic nature of phospholipid molecules. Each phospholipid molecule has a hydrophilic (water-loving) head and a hydrophobic (water-fearing) tail. When these molecules are placed in an aqueous environment, they spontaneously assemble into a bilayer structure, with the hydrophilic heads facing outwards towards the water and the hydrophobic tails facing inwards towards each other.

Amphipathic Nature of Phospholipids
The amphipathic nature of phospholipids is a critical factor in the formation of the bilayer structure. The hydrophilic head of the phospholipid molecule is composed of a phosphate group and a glycerol backbone, which are both polar and charged. This allows the head to interact with water molecules, forming hydrogen bonds and electrostatic interactions. In contrast, the hydrophobic tail is composed of fatty acid chains, which are non-polar and hydrophobic. This allows the tail to interact with other hydrophobic molecules, such as other fatty acid chains, and to avoid interacting with water molecules.
Hydrophobic Effect
The hydrophobic effect is another key factor in the formation of the phospholipid bilayer. When hydrophobic molecules are placed in an aqueous environment, they tend to aggregate and exclude water molecules from their surface. This is because the hydrophobic molecules are unable to form hydrogen bonds with water molecules, which leads to a decrease in entropy. By aggregating and excluding water molecules, the hydrophobic molecules are able to increase their entropy and reduce their free energy.
The hydrophobic effect is a major driving force behind the formation of the phospholipid bilayer. When phospholipid molecules are placed in an aqueous environment, the hydrophobic tails tend to aggregate and exclude water molecules from their surface. This leads to the formation of a bilayer structure, with the hydrophilic heads facing outwards towards the water and the hydrophobic tails facing inwards towards each other.
Entropic Favorability
The formation of the phospholipid bilayer is also entropically favorable. When phospholipid molecules are dispersed in an aqueous environment, they have a high degree of freedom and are able to move and interact with each other in a random manner. However, when the phospholipid molecules aggregate and form a bilayer structure, they become more ordered and have a lower degree of freedom. This leads to a decrease in entropy, which is entropically unfavorable.
However, the formation of the phospholipid bilayer is also accompanied by an increase in entropy of the surrounding water molecules. When the hydrophobic tails of the phospholipid molecules aggregate and exclude water molecules from their surface, the water molecules become more disordered and have a higher degree of freedom. This leads to an increase in entropy, which is entropically favorable.

Electrostatic Interactions
Electrostatic interactions between phospholipid molecules also play a critical role in the formation of the bilayer structure. The hydrophilic heads of phospholipid molecules are charged, with the phosphate group being negatively charged and the glycerol backbone being positively charged. This allows the heads to interact with each other through electrostatic interactions, such as hydrogen bonding and ionic interactions.
These electrostatic interactions help to stabilize the bilayer structure and prevent the phospholipid molecules from aggregating into a micelle or other non-bilayer structure. By interacting with each other through electrostatic interactions, the phospholipid molecules are able to maintain a stable bilayer structure, which is critical for their function in cell membranes.
Optimization of Surface Area
The final reason phospholipid bilayers form the way they do is due to the optimization of surface area. When phospholipid molecules are dispersed in an aqueous environment, they have a high surface area, which is energetically unfavorable. By aggregating and forming a bilayer structure, the phospholipid molecules are able to reduce their surface area and minimize their free energy.
The optimization of surface area is a critical factor in the formation of the phospholipid bilayer. By reducing their surface area, the phospholipid molecules are able to decrease their free energy and increase their stability. This is particularly important in cell membranes, where the phospholipid bilayer must be stable and maintain its structure over a wide range of conditions.

In conclusion, the phospholipid bilayer is a complex and fascinating structure that is critical for the function of cell membranes. The five reasons outlined above – amphipathic nature of phospholipids, hydrophobic effect, entropic favorability, electrostatic interactions, and optimization of surface area – all contribute to the formation of the bilayer structure. By understanding these factors, we can gain a deeper appreciation for the importance of the phospholipid bilayer and its role in maintaining cellular homeostasis.
We encourage you to share your thoughts and questions about phospholipid bilayers in the comments section below. How do you think the phospholipid bilayer contributes to the function of cell membranes? What other factors do you think are important for the formation and maintenance of the bilayer structure?
What is the primary component of cell membranes?
+The primary component of cell membranes is the phospholipid bilayer.
Why do phospholipid molecules aggregate into a bilayer structure?
+Phospholipid molecules aggregate into a bilayer structure due to the amphipathic nature of phospholipids, the hydrophobic effect, entropic favorability, electrostatic interactions, and optimization of surface area.
What is the role of the phospholipid bilayer in cell membranes?
+The phospholipid bilayer plays a critical role in maintaining cellular homeostasis, regulating cell signaling, and facilitating the transport of molecules across the membrane.