In the vast world of chemistry, atoms are the building blocks of matter, and how they interact with each other determines the properties of a substance. One of the fundamental concepts in chemistry is the formation of bonds between atoms. Covalent bonds, in particular, are a type of chemical bond that involves the sharing of electrons between two or more atoms. Within covalent bonds, there exists a subset known as polar covalent bonds, which are characterized by an unequal sharing of electrons. This unequal sharing leads to a molecule with a slight positive charge on one end and a slight negative charge on the other. Understanding which atoms form polar covalent bonds is crucial for grasping various chemical reactions and phenomena.
The periodic table provides a wealth of information about atoms and their tendencies to form bonds. Atoms with a high electronegativity value, which is a measure of an atom's ability to attract electrons in a covalent bond, are more likely to form polar covalent bonds. Conversely, atoms with low electronegativity values tend to lose electrons more easily, leading to the formation of ionic bonds rather than covalent bonds. The difference in electronegativity between atoms is a key factor in determining whether a covalent bond is polar or nonpolar.

In this context, let's explore five atoms that commonly form polar covalent bonds, along with examples and explanations of their bonding behaviors.
Oxygen (O)

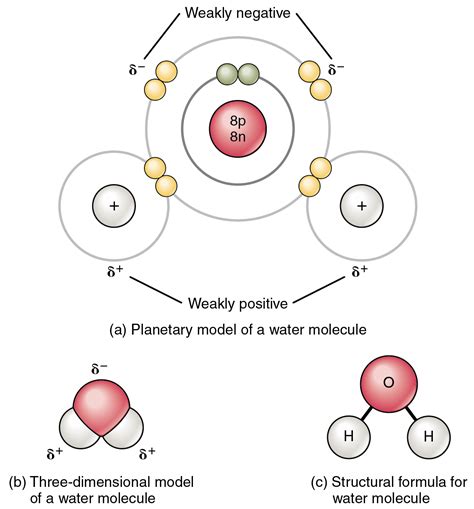
Oxygen is a highly electronegative atom, ranking 3.44 on the Pauling scale, which makes it very effective at attracting electrons in a covalent bond. This high electronegativity leads to the formation of polar covalent bonds with atoms that have significantly lower electronegativity values. A classic example is the water molecule (H2O), where oxygen forms covalent bonds with two hydrogen atoms. Due to the large difference in electronegativity between oxygen (3.44) and hydrogen (2.20), the oxygen atom pulls the shared electrons closer to itself, creating a partial negative charge on the oxygen and a partial positive charge on each hydrogen atom.
Nitrogen (N)

Nitrogen, with an electronegativity value of 3.04, also tends to form polar covalent bonds, especially with hydrogen and other less electronegative atoms. In ammonia (NH3), nitrogen forms three covalent bonds with hydrogen atoms. The unequal sharing of electrons, due to the difference in electronegativity between nitrogen and hydrogen, results in a partial positive charge on each hydrogen atom and a partial negative charge on the nitrogen atom.
Fluorine (F)

Fluorine is the most electronegative atom, with a value of 3.98 on the Pauling scale. This extreme electronegativity makes fluorine highly prone to forming polar covalent bonds. Hydrogen fluoride (HF) is a prime example, where fluorine forms a covalent bond with hydrogen. The vast difference in electronegativity between fluorine and hydrogen (2.20) leads to a highly polar bond, with fluorine having a significant partial negative charge and hydrogen having a partial positive charge.
Phosphorus (P)

Phosphorus, with an electronegativity value of 2.19, tends to form covalent bonds that are less polar compared to those formed by highly electronegative atoms like oxygen, nitrogen, and fluorine. However, when phosphorus bonds with atoms of significantly higher electronegativity, such as oxygen, it forms polar covalent bonds. In phosphoric acid (H3PO4), phosphorus forms covalent bonds with oxygen and hydrogen atoms. The bonds between phosphorus and oxygen are more polar due to the higher electronegativity of oxygen.
Sulfur (S)

Sulfur, with an electronegativity value of 2.58, is another atom that can form polar covalent bonds, particularly with hydrogen and other less electronegative atoms. Hydrogen sulfide (H2S) is an example where sulfur forms covalent bonds with hydrogen atoms. The difference in electronegativity between sulfur and hydrogen results in a polar bond, with sulfur having a partial negative charge and each hydrogen having a partial positive charge.
Understanding the formation of polar covalent bonds with these atoms is crucial for comprehending various chemical phenomena, including the properties of molecules and their reactivity. The unique characteristics of these atoms, particularly their electronegativity values, play a significant role in determining the nature of the bonds they form.
As we conclude this exploration into the world of polar covalent bonds, it's clear that the interactions between atoms are the backbone of chemistry. Understanding these interactions not only enriches our knowledge of the microscopic world but also has practical applications in fields such as biochemistry, environmental science, and materials science. We invite you to delve deeper into the fascinating realm of chemistry, where the bonds between atoms hold the secrets to understanding our world.
What determines the polarity of a covalent bond?
+The difference in electronegativity between the two atoms involved in the bond determines the polarity. A larger difference in electronegativity leads to a more polar bond.
Which atom in a polar covalent bond typically has a partial positive charge?
+The atom with the lower electronegativity value typically has a partial positive charge in a polar covalent bond.
Can atoms with similar electronegativity values form polar covalent bonds?
+Atoms with very similar electronegativity values tend to form nonpolar covalent bonds, as the difference in electronegativity is too small to result in a significant unequal sharing of electrons.