Chemical reactions are a fundamental concept in chemistry, and understanding their general form is crucial for grasping the subject. In this article, we will delve into the world of chemical reactions, exploring their definition, types, and the general form that they take.
What is a Chemical Reaction?
A chemical reaction is a process in which one or more substances, known as reactants, are converted into new substances, known as products. This process involves the breaking and forming of chemical bonds, resulting in a change in the chemical composition of the substances involved. Chemical reactions can be simple, involving only a few reactants and products, or complex, involving multiple reactants and products.

Types of Chemical Reactions
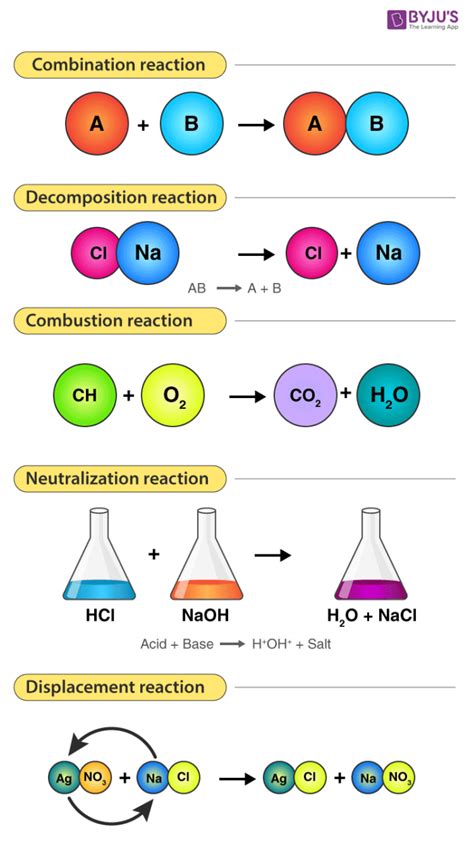
There are several types of chemical reactions, including:
- Synthesis reactions: These reactions involve the combination of two or more reactants to form a single product.
- Decomposition reactions: These reactions involve the breakdown of a single reactant into two or more products.
- Replacement reactions: These reactions involve the replacement of one reactant with another.
- Combustion reactions: These reactions involve the reaction of a substance with oxygen, resulting in the release of heat and light.
The General Form of a Chemical Reaction
The general form of a chemical reaction is:
Reactants → Products
This equation indicates that the reactants on the left-hand side of the arrow are converted into the products on the right-hand side.

Components of a Chemical Equation
A chemical equation consists of several components, including:
- Reactants: The substances that are converted into products.
- Products: The substances that are formed from the reactants.
- Arrow: The arrow indicates the direction of the reaction.
- Coefficients: Numbers that are placed in front of the reactants or products to indicate the number of molecules involved.
- Subscripts: Small numbers that are placed to the right of the chemical symbol to indicate the number of atoms of an element in a molecule.
Writing Chemical Equations
Writing chemical equations involves several steps, including:
- Identify the reactants and products.
- Write the reactants on the left-hand side of the arrow.
- Write the products on the right-hand side of the arrow.
- Balance the equation by adding coefficients and subscripts as necessary.

Examples of Chemical Reactions
Here are a few examples of chemical reactions:
- 2H2 + O2 → 2H2O (combustion reaction)
- Na + Cl2 → NaCl (synthesis reaction)
- CaCO3 → CaO + CO2 (decomposition reaction)
Conclusion
In conclusion, chemical reactions are an essential concept in chemistry, and understanding their general form is crucial for grasping the subject. By understanding the types of chemical reactions, the components of a chemical equation, and how to write chemical equations, you will be well on your way to mastering the world of chemical reactions.

Takeaways
- Chemical reactions involve the conversion of reactants into products.
- There are several types of chemical reactions, including synthesis, decomposition, replacement, and combustion reactions.
- The general form of a chemical reaction is Reactants → Products.
- Chemical equations consist of reactants, products, an arrow, coefficients, and subscripts.
- Writing chemical equations involves identifying the reactants and products, writing the reactants and products, and balancing the equation.
What's Next?
Now that you have a solid understanding of the general form of a chemical reaction, it's time to dive deeper into the world of chemistry. Stay tuned for more articles on chemical reactions, including how to balance chemical equations, the different types of chemical reactions, and more.
What is a chemical reaction?
+A chemical reaction is a process in which one or more substances, known as reactants, are converted into new substances, known as products.
What are the different types of chemical reactions?
+There are several types of chemical reactions, including synthesis, decomposition, replacement, and combustion reactions.
How do I write a chemical equation?
+Writing a chemical equation involves identifying the reactants and products, writing the reactants and products, and balancing the equation.
We hope you found this article informative and helpful. If you have any questions or comments, please feel free to ask in the comments section below.