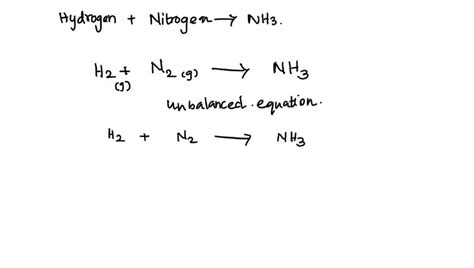
Nitrogen and hydrogen are two of the most abundant elements in the universe, and they play a crucial role in many industrial and biological processes. One of the most significant reactions involving these two elements is the Haber-Bosch process, which is used to produce ammonia gas (NH3) on a large scale. In this article, we will explore the reaction between nitrogen and hydrogen to form ammonia gas, including its importance, mechanisms, and applications.

The reaction between nitrogen and hydrogen to form ammonia gas is a complex process that involves the breaking and forming of chemical bonds. Nitrogen (N2) is a stable molecule that consists of two nitrogen atoms bonded together through a strong triple bond. Hydrogen (H2), on the other hand, is a highly reactive molecule that consists of two hydrogen atoms bonded together through a single bond. When nitrogen and hydrogen are combined in the presence of a catalyst, such as iron or ruthenium, they react to form ammonia gas according to the following equation:
N2 + 3H2 → 2NH3
This reaction is highly exothermic, releasing a significant amount of heat energy in the process. The reaction is also reversible, meaning that ammonia gas can be converted back into nitrogen and hydrogen under certain conditions.
Importance of the Reaction
The reaction between nitrogen and hydrogen to form ammonia gas is one of the most important industrial processes in the world. Ammonia is a critical component in the production of fertilizers, which are used to promote plant growth and increase crop yields. In fact, it is estimated that over 50% of the world's population relies on ammonia-based fertilizers for food production.
In addition to its use in fertilizers, ammonia is also used in a variety of other applications, including the production of plastics, textiles, and pharmaceuticals. It is also used as a refrigerant and as a fuel source in some industrial processes.

Mechanisms of the Reaction
The reaction between nitrogen and hydrogen to form ammonia gas is a complex process that involves several different mechanisms. The most widely accepted mechanism is the Haber-Bosch process, which involves the following steps:
- Nitrogen dissociation: The nitrogen molecule (N2) is dissociated into individual nitrogen atoms, which are highly reactive.
- Hydrogen dissociation: The hydrogen molecule (H2) is dissociated into individual hydrogen atoms, which are also highly reactive.
- Nitrogen-hydrogen complex formation: The individual nitrogen and hydrogen atoms combine to form a complex, which is stabilized by the catalyst.
- Ammonia formation: The nitrogen-hydrogen complex is converted into ammonia gas (NH3) through a series of complex reactions.
Catalysts and Reaction Conditions
The Haber-Bosch process requires the use of a catalyst, such as iron or ruthenium, to facilitate the reaction. The catalyst helps to stabilize the nitrogen-hydrogen complex and promote the formation of ammonia gas.
The reaction conditions for the Haber-Bosch process are typically quite severe, involving high temperatures (around 450°C) and high pressures (around 200 atm). These conditions are necessary to achieve a high yield of ammonia gas and to minimize the formation of unwanted byproducts.

Applications of the Reaction
The reaction between nitrogen and hydrogen to form ammonia gas has a wide range of applications in industry and agriculture. Some of the most significant applications include:
- Fertilizer production: Ammonia is a critical component in the production of fertilizers, which are used to promote plant growth and increase crop yields.
- Plastics production: Ammonia is used as a feedstock in the production of plastics, such as polyamide and polyurethane.
- Textile production: Ammonia is used in the production of textiles, such as nylon and polyester.
- Pharmaceutical production: Ammonia is used as a feedstock in the production of certain pharmaceuticals, such as antibiotics and vaccines.
Environmental Impact
The reaction between nitrogen and hydrogen to form ammonia gas has a significant environmental impact. The production of ammonia requires large amounts of energy, which is typically generated by burning fossil fuels and releasing greenhouse gases into the atmosphere.
In addition, the use of ammonia-based fertilizers has been linked to environmental problems, such as soil degradation and water pollution. However, many farmers and agricultural companies are now adopting more sustainable practices, such as using organic fertilizers and implementing conservation tillage.

Future Directions
The reaction between nitrogen and hydrogen to form ammonia gas is an important area of research, with many scientists and engineers working to develop more efficient and sustainable production methods.
Some of the most promising areas of research include:
- Alternative catalysts: Researchers are exploring the use of alternative catalysts, such as graphene and nanomaterials, to improve the efficiency and sustainability of the Haber-Bosch process.
- Renewable energy: Scientists are working to develop new methods for generating renewable energy, such as solar and wind power, to reduce the environmental impact of ammonia production.
- Sustainable fertilizers: Researchers are developing new types of fertilizers that are more sustainable and environmentally friendly, such as organic fertilizers and biofertilizers.

We hope this article has provided a comprehensive overview of the reaction between nitrogen and hydrogen to form ammonia gas. From its importance in industry and agriculture to its environmental impact and future directions, this reaction is a critical component of modern society.
We encourage you to share your thoughts and comments on this article, and to explore other topics related to chemistry and sustainability. Thank you for reading!
What is the Haber-Bosch process?
+The Haber-Bosch process is a method of producing ammonia gas (NH3) by reacting nitrogen (N2) and hydrogen (H2) in the presence of a catalyst.
What are the applications of ammonia gas?
+Ammonia gas has a wide range of applications, including fertilizer production, plastics production, textile production, and pharmaceutical production.
What is the environmental impact of the Haber-Bosch process?
+The Haber-Bosch process has a significant environmental impact, including the release of greenhouse gases and the use of non-renewable energy sources.