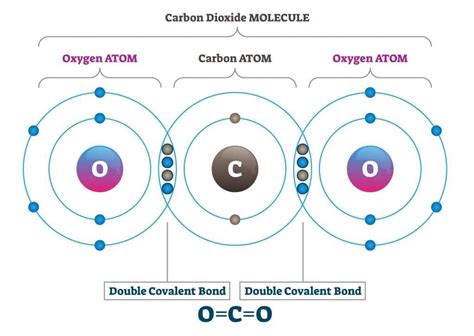
Carbon is a unique element in the periodic table, and its ability to form covalent bonds is unparalleled. This property makes carbon the basis of all life on Earth, as it is the fundamental component of biomolecules such as carbohydrates, proteins, and nucleic acids. However, have you ever wondered what limits the number of covalent bonds that a carbon atom can form?
Why Carbon Atoms Form Covalent Bonds

Carbon atoms form covalent bonds due to their unique electronic configuration. A carbon atom has six electrons in its outermost energy level, with four of these electrons being valence electrons. These valence electrons are responsible for forming bonds with other atoms. Carbon's electron configuration allows it to form four covalent bonds, which is known as tetravalency.
Limitations of Carbon Atom Covalent Bonds Formation

Although carbon atoms can form an unlimited number of covalent bonds in theory, there are several limitations that restrict the number of bonds in practice. These limitations include:
Octet Rule
The octet rule states that atoms tend to gain, lose, or share electrons to achieve a full outer energy level, which typically consists of eight electrons. Carbon atoms follow this rule, and they tend to form four covalent bonds to achieve a stable octet.
Valence Shell Electron Pair Repulsion (VSEPR) Theory
The VSEPR theory states that electron pairs in the valence shell of an atom repel each other, resulting in a tetrahedral arrangement of bonds around a carbon atom. This arrangement limits the number of covalent bonds that a carbon atom can form to four.
Atomic Size and Electronegativity
The size of a carbon atom and its electronegativity also limit the number of covalent bonds it can form. Carbon atoms are relatively small, which limits the number of bonds they can form with larger atoms. Additionally, carbon's electronegativity is moderate, which means it can form bonds with atoms that have similar electronegativities.
Consequences of Exceeding the Covalent Bond Limit

Exceeding the covalent bond limit of a carbon atom can have significant consequences, including:
Instability and Reactivity
Carbon compounds that exceed the covalent bond limit tend to be unstable and reactive. These compounds may undergo rearrangements or react with other molecules to form more stable compounds.
Distortion of Bond Angles and Lengths
Exceeding the covalent bond limit can also result in distortion of bond angles and lengths. This distortion can lead to increased reactivity and instability of the carbon compound.
Formation of Unusual Bonds
In some cases, exceeding the covalent bond limit can result in the formation of unusual bonds, such as three-center two-electron bonds or metal-carbon bonds. These bonds are often weaker and more reactive than traditional covalent bonds.
Conclusion: A New Perspective
In conclusion, the ability of carbon atoms to form covalent bonds is a unique property that underlies the diversity of organic compounds. However, there are limitations to the number of covalent bonds that a carbon atom can form, including the octet rule, VSEPR theory, atomic size, and electronegativity. Understanding these limitations can provide a new perspective on the properties and reactivity of carbon compounds.
What is the maximum number of covalent bonds that a carbon atom can form?
+A carbon atom can form a maximum of four covalent bonds.
What is the octet rule, and how does it relate to carbon atoms?
+The octet rule states that atoms tend to gain, lose, or share electrons to achieve a full outer energy level, which typically consists of eight electrons. Carbon atoms follow this rule, and they tend to form four covalent bonds to achieve a stable octet.
What happens when a carbon atom exceeds its covalent bond limit?
+Exceeding the covalent bond limit of a carbon atom can result in instability and reactivity, distortion of bond angles and lengths, and the formation of unusual bonds.