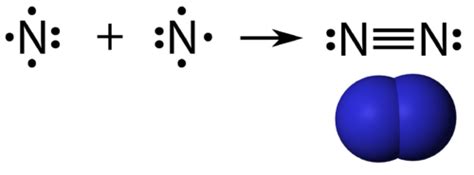Nitrogen is a fundamental element in the universe, making up a significant portion of the Earth's atmosphere and playing a crucial role in the formation of amino acids, the building blocks of life. One of the most fascinating aspects of nitrogen is its ability to form multiple bonds with other elements, a property that is essential for the creation of complex molecules.
Nitrogen's atomic structure is responsible for its unique bonding capabilities. With five valence electrons, nitrogen has a strong tendency to form covalent bonds with other atoms to achieve a stable octet configuration. This property allows nitrogen to form a wide range of compounds, from simple molecules like ammonia (NH3) to complex biomolecules like proteins and nucleic acids.
Understanding Nitrogen's Bonding Capacity

Nitrogen's bonding capacity is a result of its electronic configuration. With five valence electrons, nitrogen can form up to four covalent bonds with other atoms. This is because each covalent bond requires the sharing of one pair of electrons between two atoms. By forming four covalent bonds, nitrogen achieves a stable octet configuration, which is essential for its chemical stability.
Types of Nitrogen Bonds
Nitrogen can form several types of bonds, including:
- Single bonds: Nitrogen can form single covalent bonds with other atoms, such as hydrogen, oxygen, and carbon.
- Double bonds: Nitrogen can also form double covalent bonds with other atoms, such as oxygen and carbon.
- Triple bonds: Nitrogen can form triple covalent bonds with other atoms, such as carbon and nitrogen.
Examples of Nitrogen Compounds

Nitrogen's ability to form multiple bonds has led to the creation of a wide range of compounds, including:
- Ammonia (NH3): A simple molecule composed of one nitrogen atom bonded to three hydrogen atoms.
- Amino acids: Complex biomolecules composed of nitrogen, carbon, oxygen, and hydrogen atoms.
- Proteins: Large biomolecules composed of multiple amino acid chains bonded together through peptide bonds.
- Nucleic acids: Complex biomolecules composed of nitrogen, carbon, oxygen, and phosphorus atoms.
Importance of Nitrogen in Biological Systems
Nitrogen plays a critical role in biological systems, particularly in the formation of amino acids and proteins. Amino acids are the building blocks of proteins, which are essential for a wide range of biological processes, including enzyme catalysis, DNA replication, and cell signaling.
Nitrogen's ability to form multiple bonds has also led to the creation of complex biomolecules, such as nucleic acids, which are essential for the storage and transmission of genetic information.
Environmental Impact of Nitrogen

Nitrogen's impact on the environment is significant, particularly in the context of agriculture and climate change. The use of nitrogen-based fertilizers has led to the degradation of soil quality, the eutrophication of waterways, and the emission of greenhouse gases.
Furthermore, the burning of fossil fuels has released large amounts of nitrogen oxides into the atmosphere, contributing to air pollution and climate change.
Reducing Nitrogen's Environmental Impact
To reduce nitrogen's environmental impact, it is essential to adopt sustainable agricultural practices, such as the use of nitrogen-fixing crops and the optimization of fertilizer application.
Additionally, the development of more efficient technologies for the removal of nitrogen oxides from the atmosphere is crucial for mitigating the effects of climate change.
Conclusion
Nitrogen's ability to form multiple bonds has led to the creation of a wide range of compounds, from simple molecules like ammonia to complex biomolecules like proteins and nucleic acids.
Understanding nitrogen's bonding capacity is essential for appreciating its role in biological systems and its impact on the environment.
By adopting sustainable practices and developing more efficient technologies, we can reduce nitrogen's environmental impact and promote a healthier planet.
How many bonds can nitrogen form?
+Nitrogen can form up to four covalent bonds with other atoms.
What is the importance of nitrogen in biological systems?
+Nitrogen plays a critical role in the formation of amino acids and proteins, which are essential for a wide range of biological processes.
How can we reduce nitrogen's environmental impact?
+We can reduce nitrogen's environmental impact by adopting sustainable agricultural practices, such as the use of nitrogen-fixing crops and the optimization of fertilizer application, and by developing more efficient technologies for the removal of nitrogen oxides from the atmosphere.