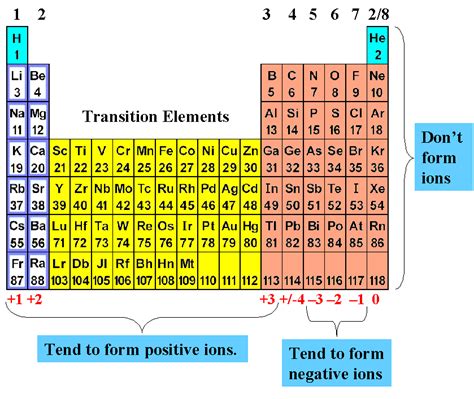
The formation of positive ions is a fundamental concept in chemistry, and it plays a crucial role in various chemical reactions and processes. Positive ions, also known as cations, are formed when an atom or a group of atoms loses one or more electrons, resulting in a net positive charge. In this article, we will explore the top 5 elements that form positive ions, along with their properties and examples.
Understanding Positive Ions
Before we dive into the top 5 elements that form positive ions, it's essential to understand the concept of positive ions. Positive ions are formed when an atom or a group of atoms loses one or more electrons, resulting in a net positive charge. This process is known as ionization. The resulting positive ion is called a cation.

Element #1: Sodium (Na)
Sodium is a highly reactive element that readily forms positive ions. When sodium loses an electron, it forms a positive ion with a charge of +1. This process is known as single ionization. Sodium ions are highly stable and are commonly found in many chemical compounds, including table salt (sodium chloride).
Properties of Sodium Ions
- Charge: +1
- Stability: Highly stable
- Examples: Table salt (sodium chloride), sodium hydroxide (NaOH)
Element #2: Magnesium (Mg)
Magnesium is another element that forms positive ions easily. When magnesium loses two electrons, it forms a positive ion with a charge of +2. This process is known as double ionization. Magnesium ions are highly reactive and are commonly found in many biological processes, including bone formation.

Properties of Magnesium Ions
- Charge: +2
- Stability: Highly reactive
- Examples: Magnesium oxide (MgO), magnesium hydroxide (Mg(OH)2)
Element #3: Aluminum (Al)
Aluminum is a highly reactive element that forms positive ions readily. When aluminum loses three electrons, it forms a positive ion with a charge of +3. This process is known as triple ionization. Aluminum ions are highly stable and are commonly found in many industrial applications, including the manufacture of aluminum foil.
Properties of Aluminum Ions
- Charge: +3
- Stability: Highly stable
- Examples: Aluminum oxide (Al2O3), aluminum hydroxide (Al(OH)3)

Element #4: Iron (Fe)
Iron is a highly reactive element that forms positive ions easily. When iron loses two or three electrons, it forms a positive ion with a charge of +2 or +3. This process is known as double or triple ionization. Iron ions are highly reactive and are commonly found in many biological processes, including the transport of oxygen in the blood.
Properties of Iron Ions
- Charge: +2 or +3
- Stability: Highly reactive
- Examples: Iron oxide (Fe2O3), iron hydroxide (Fe(OH)2)
Element #5: Calcium (Ca)
Calcium is a highly reactive element that forms positive ions readily. When calcium loses two electrons, it forms a positive ion with a charge of +2. This process is known as double ionization. Calcium ions are highly stable and are commonly found in many biological processes, including bone formation.

Properties of Calcium Ions
- Charge: +2
- Stability: Highly stable
- Examples: Calcium oxide (CaO), calcium hydroxide (Ca(OH)2)
In conclusion, the top 5 elements that form positive ions are sodium, magnesium, aluminum, iron, and calcium. These elements are highly reactive and form positive ions readily, resulting in a net positive charge. Understanding the properties and examples of these elements is essential for understanding various chemical reactions and processes.
Call to Action
We hope this article has provided you with a comprehensive understanding of the top 5 elements that form positive ions. If you have any questions or comments, please feel free to share them below. Don't forget to share this article with your friends and colleagues who may find it useful.
What is the definition of a positive ion?
+A positive ion is an atom or group of atoms that has lost one or more electrons, resulting in a net positive charge.
What is the process of forming a positive ion called?
+The process of forming a positive ion is called ionization.
What are some examples of positive ions?
+Some examples of positive ions include sodium ions (Na+), magnesium ions (Mg2+), aluminum ions (Al3+), iron ions (Fe2+ or Fe3+), and calcium ions (Ca2+).