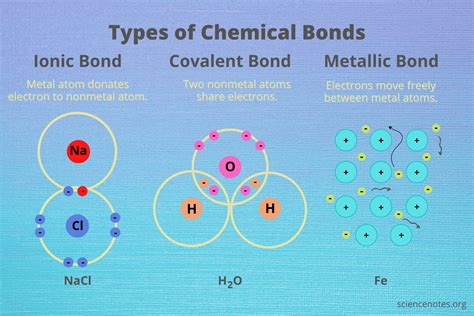
The world of chemistry is filled with fascinating phenomena, and one of the most intriguing is the way hydrogen forms ionic bonds. Ionic bonds are a type of chemical bond that occurs between two atoms that have a large difference in electronegativity, resulting in the transfer of one or more electrons from one atom to another. Hydrogen, being the lightest and most abundant element in the universe, plays a crucial role in the formation of ionic bonds.

In this article, we will delve into the three ways hydrogen forms ionic bonds, exploring the underlying mechanisms and providing examples to illustrate each process.
1. Hydrogen as a Cation
Hydrogen can form ionic bonds by losing an electron to become a positively charged ion, known as a cation. This process occurs when hydrogen is in the presence of a highly electronegative atom, such as oxygen or fluorine. The resulting hydrogen cation, H+, is highly reactive and readily forms bonds with anions, which are negatively charged ions.
For example, when hydrogen reacts with oxygen to form water (H2O), the oxygen atom pulls the shared electrons closer to itself, resulting in a partial positive charge on the hydrogen atoms. This partial positive charge allows the hydrogen atoms to form a weak ionic bond with the oxygen atom, which has a partial negative charge.
Key Factors Influencing Hydrogen Cation Formation
The formation of hydrogen cations is influenced by several factors, including:
- Electronegativity: The difference in electronegativity between hydrogen and the surrounding atom(s) plays a crucial role in determining the likelihood of hydrogen cation formation.
- pH: The pH of the surrounding environment can also affect the formation of hydrogen cations, with acidic conditions favoring the formation of H+ ions.
- Temperature: Increasing temperature can also promote the formation of hydrogen cations, as it provides the necessary energy for the reaction to occur.

2. Hydrogen as an Anion
In addition to forming cations, hydrogen can also form anions by gaining an electron to become a negatively charged ion. This process occurs when hydrogen is in the presence of a highly electropositive atom, such as alkali metals like sodium or potassium.
For example, when hydrogen reacts with sodium to form sodium hydride (NaH), the sodium atom donates an electron to the hydrogen atom, resulting in the formation of a hydrogen anion, H-. This anion can then form ionic bonds with other cations, such as sodium ions.
Key Factors Influencing Hydrogen Anion Formation
The formation of hydrogen anions is influenced by several factors, including:
- Electropositivity: The electropositivity of the surrounding atom(s) plays a crucial role in determining the likelihood of hydrogen anion formation.
- Electron affinity: The electron affinity of the surrounding atom(s) also affects the formation of hydrogen anions, with atoms having a high electron affinity favoring the formation of H- ions.
- Pressure: Increasing pressure can also promote the formation of hydrogen anions, as it provides the necessary energy for the reaction to occur.

3. Hydrogen as a Hydrogen Bond Donor
Hydrogen can also form ionic bonds by acting as a hydrogen bond donor. Hydrogen bonding occurs when a hydrogen atom bonded to a highly electronegative atom, such as oxygen or nitrogen, forms a weak bond with another electronegative atom.
For example, when hydrogen is bonded to oxygen in a water molecule, the hydrogen atom can form a hydrogen bond with another oxygen atom in a neighboring water molecule. This hydrogen bond is weaker than a covalent bond but stronger than a van der Waals interaction.
Key Factors Influencing Hydrogen Bond Formation
The formation of hydrogen bonds is influenced by several factors, including:
- Electronegativity: The electronegativity of the surrounding atom(s) plays a crucial role in determining the likelihood of hydrogen bond formation.
- Distance: The distance between the hydrogen atom and the electronegative atom(s) also affects the formation of hydrogen bonds, with shorter distances favoring the formation of stronger bonds.
- Temperature: Increasing temperature can also affect the formation of hydrogen bonds, with higher temperatures favoring the breaking of these bonds.

In conclusion, hydrogen forms ionic bonds through three distinct mechanisms: as a cation, as an anion, and as a hydrogen bond donor. Understanding these mechanisms is crucial for grasping the complex behavior of hydrogen in various chemical environments. Whether you're a chemist, a physicist, or simply a curious learner, the fascinating world of hydrogen ionic bonds is sure to captivate and inspire.
We'd love to hear from you! Share your thoughts on the importance of hydrogen ionic bonds in the comments below. What do you think is the most significant application of hydrogen ionic bonds? Let's continue the conversation!
What is the difference between a hydrogen cation and a hydrogen anion?
+A hydrogen cation (H+) is a positively charged ion formed when hydrogen loses an electron, while a hydrogen anion (H-) is a negatively charged ion formed when hydrogen gains an electron.
What is the role of electronegativity in hydrogen ionic bond formation?
+Electronegativity plays a crucial role in determining the likelihood of hydrogen ionic bond formation. Highly electronegative atoms can pull electrons closer to themselves, resulting in the formation of hydrogen cations or anions.
What is the difference between a hydrogen bond and a covalent bond?
+A hydrogen bond is a weak electrostatic attraction between a hydrogen atom bonded to a highly electronegative atom and another electronegative atom, while a covalent bond is a stronger chemical bond formed through the sharing of electrons between atoms.