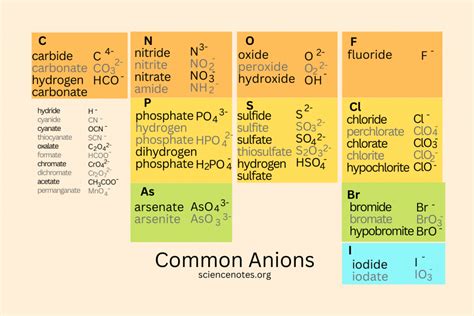
Nonmetals are a group of elements in the periodic table that are characterized by their ability to gain electrons to form anions. This ability is a key factor in their chemical properties and reactivity. But what are the reasons behind nonmetals forming anions? In this article, we will explore the five main reasons why nonmetals tend to form anions.
Why Nonmetals Form Anions

Reason 1: Electron Configuration
Nonmetals have a tendency to gain electrons to achieve a full outer energy level. This is because their electron configuration is such that they have a relatively small number of electrons in their outermost energy level. By gaining electrons, nonmetals can achieve a more stable electron configuration, which is a key factor in their ability to form anions.
For example, the nonmetal oxygen has six electrons in its outermost energy level. By gaining two electrons, oxygen can achieve a full outer energy level, resulting in the formation of the oxide anion (O2-).
Reason 2: Electronegativity
Nonmetals tend to have high electronegativity values, which means they have a strong tendency to attract electrons towards themselves. This high electronegativity is due to the fact that nonmetals have a relatively small atomic size and a high number of protons in their atomic nucleus.
As a result of their high electronegativity, nonmetals are able to pull electrons towards themselves, resulting in the formation of anions. For example, the nonmetal fluorine has a high electronegativity value of 3.98, which allows it to form the fluoride anion (F-) by pulling an electron towards itself.
Reason 3: Ionization Energy
Nonmetals tend to have high ionization energies, which means they require a lot of energy to remove an electron from their outermost energy level. However, when nonmetals do gain electrons, their ionization energy decreases, allowing them to form anions more easily.
For example, the nonmetal chlorine has a high ionization energy of 1215 kJ/mol. However, when chlorine gains an electron to form the chloride anion (Cl-), its ionization energy decreases to 348 kJ/mol, making it more stable.
Reason 4: Electron Affinity
Nonmetals tend to have high electron affinities, which means they have a strong tendency to attract electrons towards themselves. This high electron affinity is due to the fact that nonmetals have a relatively small atomic size and a high number of protons in their atomic nucleus.
As a result of their high electron affinity, nonmetals are able to attract electrons towards themselves, resulting in the formation of anions. For example, the nonmetal sulfur has a high electron affinity of 200 kJ/mol, which allows it to form the sulfide anion (S2-) by attracting two electrons towards itself.
Reason 5: Chemical Reactivity
Nonmetals tend to be highly reactive, which means they readily form chemical bonds with other elements. This high reactivity is due to the fact that nonmetals have a relatively small number of electrons in their outermost energy level, making it easy for them to gain or lose electrons.
As a result of their high reactivity, nonmetals are able to form anions by reacting with other elements. For example, the nonmetal carbon can react with oxygen to form the carbonate anion (CO32-).
Examples of Nonmetals Forming Anions

- Oxygen (O2-) forms an anion by gaining two electrons to achieve a full outer energy level.
- Fluorine (F-) forms an anion by pulling an electron towards itself due to its high electronegativity.
- Chlorine (Cl-) forms an anion by gaining an electron to decrease its ionization energy.
- Sulfur (S2-) forms an anion by attracting two electrons towards itself due to its high electron affinity.
- Carbon (CO32-) forms an anion by reacting with oxygen to form the carbonate anion.
Conclusion
In conclusion, nonmetals form anions due to a combination of factors, including their electron configuration, electronegativity, ionization energy, electron affinity, and chemical reactivity. Understanding these reasons is crucial in predicting the behavior of nonmetals in chemical reactions and in explaining the properties of anions.
We hope this article has helped you understand why nonmetals form anions. If you have any questions or comments, please feel free to share them below.
What is an anion?
+An anion is a negatively charged ion that is formed when an atom or group of atoms gains one or more electrons.
Why do nonmetals tend to form anions?
+Nonmetals tend to form anions due to a combination of factors, including their electron configuration, electronegativity, ionization energy, electron affinity, and chemical reactivity.
What is electronegativity?
+Electronegativity is a measure of an atom's ability to attract electrons towards itself. Nonmetals tend to have high electronegativity values, which allows them to form anions.