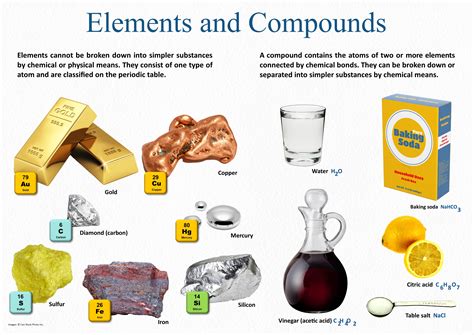
The world of chemistry is full of fascinating phenomena, and one of the most intriguing aspects is the way elements form compounds. Compounds are substances formed when two or more different elements are chemically bonded together. In this article, we will explore the five main reasons why elements form compounds.
Reason 1: The Octet Rule

Example: Sodium and Chlorine
Sodium (Na) has one electron in its outermost energy level, while chlorine (Cl) has seven electrons. When sodium and chlorine react, sodium loses an electron to form a positive ion (Na+), while chlorine gains an electron to form a negative ion (Cl-). The electrostatic attraction between the oppositely charged ions forms a strong ionic bond, resulting in the compound sodium chloride (NaCl).Reason 2: Electrostatic Attraction

Example: Calcium and Oxygen
Calcium (Ca) has two electrons in its outermost energy level, while oxygen (O) has six electrons. When calcium and oxygen react, calcium loses two electrons to form a positive ion (Ca2+), while oxygen gains two electrons to form a negative ion (O2-). The electrostatic attraction between the oppositely charged ions forms a strong ionic bond, resulting in the compound calcium oxide (CaO).Reason 3: Shared Electrons

Example: Hydrogen and Oxygen
Hydrogen (H) has one electron in its outermost energy level, while oxygen (O) has six electrons. When hydrogen and oxygen react, they share a pair of electrons to form a covalent bond, resulting in the compound water (H2O).Reason 4: Metal-Ligand Interactions

Example: Iron and Oxygen
Iron (Fe) has two electrons in its outermost energy level, while oxygen (O) has six electrons. When iron and oxygen react, iron forms a complex with oxygen, resulting in the compound iron oxide (FeO).Reason 5: Thermodynamic Stability

Example: Carbon and Hydrogen
Carbon (C) has four electrons in its outermost energy level, while hydrogen (H) has one electron. When carbon and hydrogen react, they form a covalent bond, resulting in the compound methane (CH4). The energy released during this reaction is greater than the energy required to break the bonds between the carbon and hydrogen atoms, making methane a more stable compound than its individual elements.In conclusion, the formation of compounds is a complex process driven by multiple factors, including the octet rule, electrostatic attraction, shared electrons, metal-ligand interactions, and thermodynamic stability. Understanding these factors is essential for understanding the properties and behavior of compounds, which is crucial for many applications in chemistry, biology, and industry.
We hope this article has provided a comprehensive overview of the reasons why elements form compounds. Share your thoughts and comments below, and don't forget to share this article with your friends and colleagues!
What is the octet rule?
+The octet rule states that atoms tend to gain, lose, or share electrons to achieve a full outer energy level, which typically consists of eight electrons.
What is electrostatic attraction?
+Electrostatic attraction is a fundamental force that drives the formation of compounds, where oppositely charged ions are attracted to each other, resulting in the formation of strong ionic bonds.
What is thermodynamic stability?
+Thermodynamic stability is a measure of the energy change associated with the formation of a compound, where compounds are often more stable than their individual elements.