The fascinating world of chemistry is full of intricate relationships and reactions. One of the fundamental concepts in chemistry is the ionic bond, which is a type of chemical bond that forms between two atoms that have opposite charges. This bond is essential for the formation of many compounds, including table salt (sodium chloride) and calcium carbonate (limestone). In this article, we will delve into the world of ionic bonds and explore the five elements that commonly form these bonds.

What are Ionic Bonds?
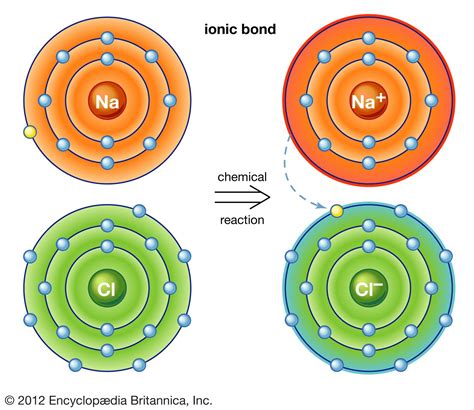
Ionic bonds are a type of chemical bond that forms between two atoms that have opposite charges. This bond is typically formed between a metal atom and a non-metal atom. The metal atom loses one or more electrons to form a positively charged ion, known as a cation. The non-metal atom gains one or more electrons to form a negatively charged ion, known as an anion. The electrostatic attraction between the oppositely charged ions holds them together and forms a strong chemical bond.
How are Ionic Bonds Formed?
Ionic bonds are formed through a process known as electron transfer. When a metal atom loses an electron, it becomes a positively charged ion. This positively charged ion is attracted to a negatively charged ion, which is formed when a non-metal atom gains an electron. The electrostatic attraction between the two ions holds them together and forms a strong chemical bond.
5 Elements That Form Ionic Bonds
Now that we have a basic understanding of ionic bonds, let's take a look at five elements that commonly form these bonds.
1. Sodium (Na)
Sodium is a highly reactive metal that readily loses an electron to form a positively charged ion. This makes it an ideal candidate for forming ionic bonds with non-metal atoms like chlorine and oxygen.

2. Calcium (Ca)
Calcium is another metal that readily loses electrons to form a positively charged ion. This makes it an ideal candidate for forming ionic bonds with non-metal atoms like oxygen and carbon.

3. Chlorine (Cl)
Chlorine is a highly reactive non-metal that readily gains an electron to form a negatively charged ion. This makes it an ideal candidate for forming ionic bonds with metal atoms like sodium and potassium.

4. Oxygen (O)
Oxygen is a highly reactive non-metal that readily gains an electron to form a negatively charged ion. This makes it an ideal candidate for forming ionic bonds with metal atoms like sodium and calcium.

5. Potassium (K)
Potassium is a highly reactive metal that readily loses an electron to form a positively charged ion. This makes it an ideal candidate for forming ionic bonds with non-metal atoms like chlorine and oxygen.

Importance of Ionic Bonds
Ionic bonds play a crucial role in the formation of many compounds, including table salt (sodium chloride) and calcium carbonate (limestone). These compounds are essential for many industrial and biological processes.
Industrial Applications
Ionic bonds are used in many industrial applications, including the production of paper, textiles, and pharmaceuticals.
Biological Applications
Ionic bonds are essential for many biological processes, including the transmission of nerve impulses and the contraction of muscles.
Conclusion
In conclusion, ionic bonds are a type of chemical bond that forms between two atoms that have opposite charges. The five elements that commonly form these bonds are sodium, calcium, chlorine, oxygen, and potassium. These elements are essential for the formation of many compounds, including table salt and calcium carbonate. Understanding the properties and behavior of these elements is crucial for many industrial and biological processes.
We hope this article has provided you with a comprehensive understanding of ionic bonds and the elements that form them. If you have any questions or comments, please feel free to share them with us.
What is an ionic bond?
+An ionic bond is a type of chemical bond that forms between two atoms that have opposite charges.
What are the five elements that commonly form ionic bonds?
+The five elements that commonly form ionic bonds are sodium, calcium, chlorine, oxygen, and potassium.
What is the importance of ionic bonds?
+