Ionic bonds are a type of chemical bond that forms between two atoms that have a large difference in electronegativity. This type of bond is typically formed between a metal and a nonmetal atom. In this article, we will discuss four ways that ionic bonds can form.

What are Ionic Bonds?
Ionic bonds are a type of chemical bond that forms when one or more electrons are transferred from one atom to another. This transfer of electrons leads to the formation of ions with opposite charges, which are then attracted to each other. The electrostatic attraction between the oppositely charged ions is what holds them together and forms the ionic bond.
Properties of Ionic Bonds
Ionic bonds have several distinct properties that distinguish them from other types of chemical bonds. These properties include:
- High melting and boiling points: Ionic compounds typically have high melting and boiling points due to the strong electrostatic attraction between the ions.
- Hardness: Ionic compounds are often hard and brittle due to the strong bonds between the ions.
- Conductivity: Ionic compounds are often good conductors of electricity when dissolved in water or melted.
- Solubility: Ionic compounds are often soluble in water due to the ability of the ions to interact with water molecules.
Formation of Ionic Bonds through Electron Transfer
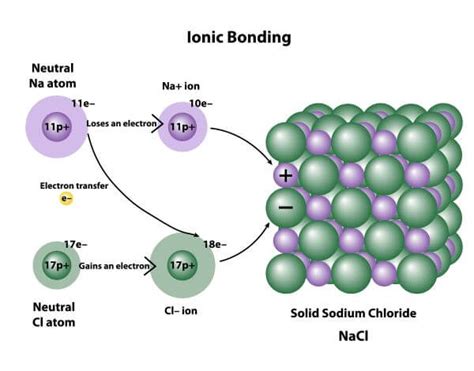
One way that ionic bonds can form is through the transfer of electrons from one atom to another. This process typically occurs between a metal atom and a nonmetal atom. The metal atom loses one or more electrons to form a positively charged ion, while the nonmetal atom gains one or more electrons to form a negatively charged ion.

For example, consider the reaction between sodium (Na) and chlorine (Cl):
Na → Na+ + e- Cl + e- → Cl-
In this reaction, the sodium atom loses an electron to form a positively charged sodium ion, while the chlorine atom gains an electron to form a negatively charged chloride ion. The electrostatic attraction between the oppositely charged ions forms the ionic bond.
Formation of Ionic Bonds through Ionization Energy
Another way that ionic bonds can form is through the ionization energy of the atoms involved. Ionization energy is the energy required to remove an electron from an atom. When the ionization energy of a metal atom is low, it is easy for the atom to lose an electron and form a positively charged ion.

For example, consider the reaction between potassium (K) and oxygen (O):
K → K+ + e- O + e- → O2-
In this reaction, the potassium atom has a low ionization energy, making it easy for the atom to lose an electron and form a positively charged potassium ion. The oxygen atom has a high electron affinity, making it easy for the atom to gain an electron and form a negatively charged oxide ion.
Formation of Ionic Bonds through Electron Affinity
A third way that ionic bonds can form is through the electron affinity of the atoms involved. Electron affinity is the energy released when an electron is added to an atom. When the electron affinity of a nonmetal atom is high, it is easy for the atom to gain an electron and form a negatively charged ion.

For example, consider the reaction between calcium (Ca) and fluorine (F):
Ca → Ca2+ + 2e- F + e- → F-
In this reaction, the fluorine atom has a high electron affinity, making it easy for the atom to gain an electron and form a negatively charged fluoride ion. The calcium atom loses two electrons to form a positively charged calcium ion.
Formation of Ionic Bonds through Lattice Energy
A fourth way that ionic bonds can form is through the lattice energy of the ions involved. Lattice energy is the energy released when ions come together to form a solid lattice. When the lattice energy of the ions is high, it is easy for the ions to come together and form a solid ionic compound.

For example, consider the reaction between sodium (Na) and chloride (Cl):
Na+ + Cl- → NaCl
In this reaction, the sodium ion and chloride ion come together to form a solid lattice of sodium chloride. The lattice energy of the ions is high, making it easy for the ions to come together and form a solid ionic compound.
We hope this article has helped you understand the different ways that ionic bonds can form. Whether it's through electron transfer, ionization energy, electron affinity, or lattice energy, ionic bonds play a crucial role in the formation of many different types of compounds. By understanding how ionic bonds form, you can better appreciate the complex chemistry that underlies many of the substances we use every day.
What is an ionic bond?
+An ionic bond is a type of chemical bond that forms between two atoms that have a large difference in electronegativity.
How do ionic bonds form?
+Ionic bonds can form through electron transfer, ionization energy, electron affinity, and lattice energy.
What are some examples of ionic compounds?
+Some examples of ionic compounds include sodium chloride (NaCl), calcium carbonate (CaCO3), and potassium nitrate (KNO3).