The periodic table is a powerful tool that helps us understand the properties and behavior of elements. One of the key concepts in chemistry is the formation of ions, which are atoms or molecules that have gained or lost electrons to form charged particles. In this article, we will explore which group forms 1+ ions easily in chemistry.
Importance of Ion Formation
Ion formation is a fundamental process in chemistry that plays a crucial role in many chemical reactions. Ions are formed when an atom or molecule gains or loses electrons to achieve a more stable electronic configuration. This process is essential for many biological and chemical processes, including the formation of compounds, the transfer of energy, and the maintenance of pH balance.
Groups in the Periodic Table
The periodic table is arranged in a way that elements with similar properties and electron configurations are placed in the same group. The groups are numbered from 1 to 18, and each group has its unique characteristics. Some groups are more prone to forming ions than others.
Group 1 Elements: The Alkali Metals
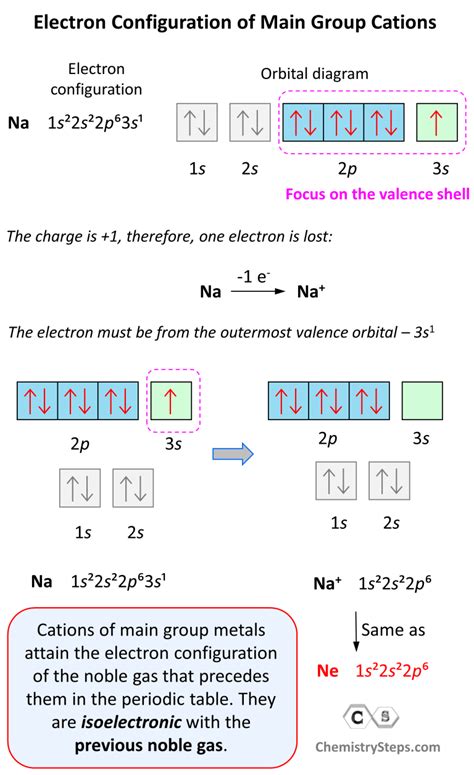
Group 1 elements, also known as the alkali metals, are located in the first column of the periodic table. These elements include lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr). The alkali metals are known for their ability to form 1+ ions easily.

Reasons for Easy Ion Formation
So, why do group 1 elements form 1+ ions easily? There are several reasons for this:
Low Ionization Energy
Group 1 elements have low ionization energy, which means that it takes less energy to remove an electron from the atom. This makes it easier for the element to lose an electron and form a positive ion.
Single Electron in the Outermost Shell
Group 1 elements have a single electron in their outermost shell, which is easily lost to form a positive ion. This single electron is not strongly attracted to the nucleus, making it easier to remove.
High Reactivity
Group 1 elements are highly reactive, which means that they readily lose electrons to form ions. This reactivity is due to the low ionization energy and the single electron in the outermost shell.
Examples of Ion Formation
Let's look at some examples of how group 1 elements form 1+ ions:
- Sodium (Na) loses an electron to form a sodium ion (Na+): Na → Na+ + e-
- Potassium (K) loses an electron to form a potassium ion (K+): K → K+ + e-
- Lithium (Li) loses an electron to form a lithium ion (Li+): Li → Li+ + e-

Other Groups that Form 1+ Ions
While group 1 elements are the most well-known for forming 1+ ions, other groups can also form ions with a +1 charge. These groups include:
- Group 2 elements (the alkaline earth metals), which form 2+ ions, but can also form 1+ ions in some cases.
- Group 13 elements (the boron group), which can form 1+ ions, but this is less common than for group 1 elements.

Conclusion
In conclusion, group 1 elements are the most likely to form 1+ ions easily in chemistry. This is due to their low ionization energy, single electron in the outermost shell, and high reactivity. Understanding ion formation is essential for many chemical processes, and recognizing the groups that form ions easily can help us predict and explain chemical reactions.

We hope this article has helped you understand which group forms 1+ ions easily in chemistry. If you have any questions or comments, please feel free to share them with us.
What is ion formation in chemistry?
+Ions are formed when an atom or molecule gains or loses electrons to form charged particles.
Which group forms 1+ ions easily in chemistry?
+Group 1 elements, also known as the alkali metals, form 1+ ions easily in chemistry.
Why do group 1 elements form 1+ ions easily?
+Group 1 elements have low ionization energy, a single electron in the outermost shell, and high reactivity, making it easy for them to lose an electron and form a positive ion.