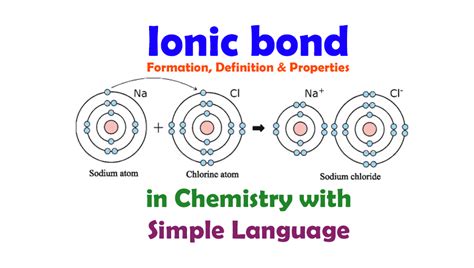
Ionic bonds are a type of chemical bond that involves the electrostatic attraction between oppositely charged ions. This type of bond is typically formed between a metal and a nonmetal. In this article, we will explore the top 5 elements that form ionic bonds with metals.

What are Ionic Bonds?
Ionic bonds are a type of chemical bond that involves the transfer of electrons between atoms. This type of bond is typically formed between a metal and a nonmetal. The metal atom loses one or more electrons to form a positively charged ion, while the nonmetal atom gains one or more electrons to form a negatively charged ion. The electrostatic attraction between the oppositely charged ions holds them together, forming an ionic bond.
How are Ionic Bonds Formed?
Ionic bonds are formed through a process called electron transfer. This process involves the transfer of electrons from the metal atom to the nonmetal atom. The metal atom loses one or more electrons to form a positively charged ion, while the nonmetal atom gains one or more electrons to form a negatively charged ion. The electrostatic attraction between the oppositely charged ions holds them together, forming an ionic bond.
Top 5 Elements that Form Ionic Bonds with Metals
There are several elements that form ionic bonds with metals. Here are the top 5 elements that form ionic bonds with metals:

1. Oxygen (O)
Oxygen is one of the most common elements that form ionic bonds with metals. Oxygen is a highly reactive nonmetal that readily forms ions with metals. Many metal oxides, such as iron oxide and aluminum oxide, are formed through ionic bonds between oxygen and metal ions.

2. Chlorine (Cl)
Chlorine is another highly reactive nonmetal that forms ionic bonds with metals. Chlorine readily forms ions with metals, resulting in the formation of metal chlorides, such as sodium chloride and calcium chloride.

3. Fluorine (F)
Fluorine is a highly reactive nonmetal that forms ionic bonds with metals. Fluorine readily forms ions with metals, resulting in the formation of metal fluorides, such as sodium fluoride and calcium fluoride.

4. Nitrogen (N)
Nitrogen is a nonmetal that forms ionic bonds with metals. Nitrogen readily forms ions with metals, resulting in the formation of metal nitrates, such as potassium nitrate and calcium nitrate.

5. Sulfur (S)
Sulfur is a nonmetal that forms ionic bonds with metals. Sulfur readily forms ions with metals, resulting in the formation of metal sulfates, such as copper sulfate and zinc sulfate.

Conclusion
In conclusion, ionic bonds are a type of chemical bond that involves the electrostatic attraction between oppositely charged ions. The top 5 elements that form ionic bonds with metals are oxygen, chlorine, fluorine, nitrogen, and sulfur. These elements readily form ions with metals, resulting in the formation of a wide range of compounds.
We hope this article has provided you with a better understanding of ionic bonds and the elements that form them. If you have any questions or comments, please feel free to share them below.
What is an ionic bond?
+An ionic bond is a type of chemical bond that involves the electrostatic attraction between oppositely charged ions.
How are ionic bonds formed?
+Ionic bonds are formed through a process called electron transfer, which involves the transfer of electrons from the metal atom to the nonmetal atom.
What are the top 5 elements that form ionic bonds with metals?
+The top 5 elements that form ionic bonds with metals are oxygen, chlorine, fluorine, nitrogen, and sulfur.