The unique properties of polyclonal antiserum have fascinated researchers for decades. One of the most intriguing aspects of polyclonal antiserum is its ability to form precipitin, a crucial component in various immunological reactions. But what sets polyclonal antiserum apart from other types of antiserum, and how does it form precipitin uniquely? In this article, we will delve into the world of immunology and explore the 5 reasons why polyclonal antiserum forms precipitin uniquely.
Understanding Polyclonal Antiserum and Precipitin

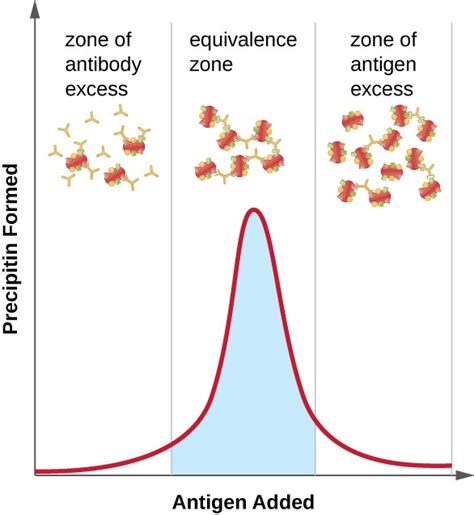
Before we dive into the reasons behind polyclonal antiserum's unique precipitin formation, it's essential to understand what polyclonal antiserum and precipitin are. Polyclonal antiserum is a type of antiserum that contains a mixture of different antibodies, each produced by a distinct B-cell clone. These antibodies are directed against various epitopes on an antigen, resulting in a diverse and heterogeneous immune response. Precipitin, on the other hand, is a type of immune complex that forms when antibodies bind to antigens, leading to the precipitation of the antigen-antibody complex.
Reason 1: Heterogeneous Antibody Population
One of the primary reasons polyclonal antiserum forms precipitin uniquely is due to its heterogeneous antibody population. Unlike monoclonal antiserum, which contains antibodies produced by a single B-cell clone, polyclonal antiserum contains a mixture of antibodies from multiple B-cell clones. This diversity of antibodies allows polyclonal antiserum to recognize and bind to multiple epitopes on an antigen, increasing the likelihood of precipitin formation.
Diversity of Epitope Recognition

The diversity of epitope recognition is a critical factor in polyclonal antiserum's unique precipitin formation. With multiple antibodies recognizing different epitopes on an antigen, the likelihood of cross-linking and precipitin formation increases. This diversity also allows polyclonal antiserum to respond to a broader range of antigens, making it a valuable tool in various immunological applications.
Reason 2: Increased Cross-Linking
The heterogeneous antibody population in polyclonal antiserum also leads to increased cross-linking, which is essential for precipitin formation. When multiple antibodies bind to different epitopes on an antigen, they can cross-link, forming a network of antigen-antibody complexes. This cross-linking increases the size and complexity of the immune complex, leading to the precipitation of the antigen-antibody complex.
Antibody-Antigen Interactions

Antibody-antigen interactions play a crucial role in polyclonal antiserum's unique precipitin formation. The strength and specificity of these interactions determine the likelihood of precipitin formation. Polyclonal antiserum's heterogeneous antibody population allows for a broader range of antibody-antigen interactions, increasing the likelihood of precipitin formation.
Reason 3: Enhanced Affinity Maturation
Affinity maturation is a process by which B-cells undergo somatic hypermutation and selection, leading to the production of high-affinity antibodies. Polyclonal antiserum's heterogeneous antibody population undergoes enhanced affinity maturation, resulting in the production of high-affinity antibodies that are more likely to form precipitin.
Affinity Maturation and Precipitin Formation

The relationship between affinity maturation and precipitin formation is complex and multifaceted. High-affinity antibodies are more likely to form precipitin due to their increased binding strength and specificity. Polyclonal antiserum's enhanced affinity maturation leads to the production of high-affinity antibodies that are more likely to form precipitin.
Reason 4: Increased Valency
The valency of an antibody refers to the number of binding sites it has for an antigen. Polyclonal antiserum's heterogeneous antibody population has increased valency due to the presence of multiple antibodies with different binding sites. This increased valency allows polyclonal antiserum to bind to multiple epitopes on an antigen, increasing the likelihood of precipitin formation.
Valency and Precipitin Formation

The relationship between valency and precipitin formation is critical in understanding polyclonal antiserum's unique properties. Increased valency allows polyclonal antiserum to bind to multiple epitopes on an antigen, leading to the formation of large immune complexes that can precipitate.
Reason 5: Synergistic Effects
The final reason polyclonal antiserum forms precipitin uniquely is due to the synergistic effects of its heterogeneous antibody population. The combination of multiple antibodies with different binding sites, increased cross-linking, enhanced affinity maturation, and increased valency creates a synergistic effect that increases the likelihood of precipitin formation.
Conclusion

In conclusion, polyclonal antiserum's unique properties make it an invaluable tool in various immunological applications. Its heterogeneous antibody population, increased cross-linking, enhanced affinity maturation, increased valency, and synergistic effects all contribute to its ability to form precipitin uniquely. Understanding these properties is essential in harnessing the power of polyclonal antiserum in various immunological applications.
Now that you've read this article, we invite you to share your thoughts and comments on the unique properties of polyclonal antiserum. How do you think polyclonal antiserum's properties can be harnessed in various immunological applications? Share your insights and let's start a conversation!
What is polyclonal antiserum?
+Polyclonal antiserum is a type of antiserum that contains a mixture of different antibodies, each produced by a distinct B-cell clone.
What is precipitin?
+Precipitin is a type of immune complex that forms when antibodies bind to antigens, leading to the precipitation of the antigen-antibody complex.
What are the unique properties of polyclonal antiserum?
+Polyclonal antiserum has a heterogeneous antibody population, increased cross-linking, enhanced affinity maturation, increased valency, and synergistic effects, all of which contribute to its unique ability to form precipitin.