The periodic table is a powerful tool that helps us understand the properties and behavior of elements. One of the key concepts in chemistry is electron configuration, which describes the arrangement of electrons in an atom. In this article, we will delve into the neon electron configuration, exploring its step-by-step breakdown and significance in chemistry.

Understanding electron configuration is crucial in chemistry, as it helps us predict the properties and behavior of elements. Electron configuration is the arrangement of electrons in an atom, which is determined by the number of protons in the nucleus. The number of protons in the nucleus is equal to the atomic number of the element.
The neon atom has 10 protons in its nucleus, which means it has an atomic number of 10. To determine the electron configuration of neon, we need to follow a step-by-step process.
Step 1: Determine the Number of Electrons
The first step in determining the electron configuration of neon is to calculate the number of electrons in the atom. Since neon has an atomic number of 10, it means it has 10 protons in its nucleus. To maintain neutrality, the number of electrons in the atom must be equal to the number of protons. Therefore, neon has 10 electrons.

Step 2: Understand the Electron Shells
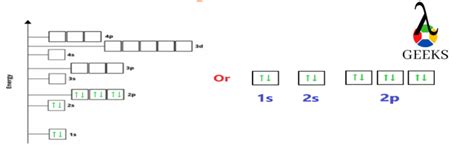
Electrons in an atom are arranged in energy levels or electron shells. Each electron shell has a specific capacity, and electrons occupy the lowest available energy levels. The first electron shell, also known as the 1s orbital, can hold up to 2 electrons. The second electron shell, which includes the 2s and 2p orbitals, can hold up to 8 electrons.
Step 2.1: Fill the 1s Orbital
The first step in filling the electron shells is to fill the 1s orbital. The 1s orbital can hold up to 2 electrons, and since neon has 10 electrons, we can fill the 1s orbital with 2 electrons.

Step 2.2: Fill the 2s Orbital
After filling the 1s orbital, the next step is to fill the 2s orbital. The 2s orbital can hold up to 2 electrons, and since we have 8 electrons remaining, we can fill the 2s orbital with 2 electrons.

Step 2.3: Fill the 2p Orbitals
The next step is to fill the 2p orbitals. The 2p orbitals can hold up to 6 electrons, and since we have 6 electrons remaining, we can fill the 2p orbitals with 6 electrons.

Step 3: Write the Electron Configuration
Now that we have filled the electron shells, we can write the electron configuration of neon. The electron configuration is written in a specific format, with the number of electrons in each orbital separated by commas.
The electron configuration of neon is:
1s² 2s² 2p⁶

Significance of Neon Electron Configuration
The neon electron configuration is significant in chemistry because it helps us understand the properties and behavior of neon. Neon is a noble gas, which means it is unreactive and has a full outer energy level. The electron configuration of neon explains why it is unreactive and why it has a full outer energy level.

Practical Applications of Neon Electron Configuration
The neon electron configuration has several practical applications in chemistry and physics. One of the most significant applications is in the production of neon signs. Neon signs are made by exciting neon atoms with electricity, which causes them to emit light.

Conclusion and Future Directions
In conclusion, the neon electron configuration is a fundamental concept in chemistry that helps us understand the properties and behavior of neon. By following a step-by-step process, we can determine the electron configuration of neon and understand its significance in chemistry.
As we continue to explore the properties and behavior of elements, it is essential to understand the electron configuration of each element. By doing so, we can gain a deeper understanding of the periodic table and the behavior of elements.

We hope this article has provided a comprehensive overview of the neon electron configuration. If you have any questions or comments, please feel free to share them below.
FAQ Section:
What is the atomic number of neon?
+The atomic number of neon is 10.
What is the electron configuration of neon?
+The electron configuration of neon is 1s² 2s² 2p⁶.
Why is neon unreactive?
+Neon is unreactive because it has a full outer energy level, which means it has a stable electron configuration.