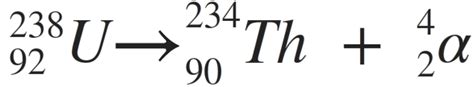Uranium 238 is a naturally occurring radioactive element that undergoes a process called radioactive decay, resulting in the formation of thorium 234. This process is a crucial part of the nuclear fuel cycle and has significant implications for nuclear energy, medicine, and scientific research.
The decay of uranium 238 into thorium 234 occurs through a series of complex nuclear reactions, involving the emission of alpha particles and the conversion of one element into another. In this article, we will delve into the details of this process, exploring the underlying mechanisms, the role of radioactive decay, and the importance of uranium 238 and thorium 234 in various fields.
Understanding Radioactive Decay
Radioactive decay is a spontaneous process in which unstable atoms lose energy and stability by emitting radiation. This process can occur through various mechanisms, including alpha decay, beta decay, and gamma decay. Alpha decay is the most common form of radioactive decay, where an unstable nucleus emits an alpha particle (two protons and two neutrons) to form a more stable nucleus.

The Decay Chain of Uranium 238
Uranium 238 is a naturally occurring radioactive element with an atomic number of 92 and an atomic mass of 238. It undergoes a series of radioactive decays, resulting in the formation of thorium 234. The decay chain of uranium 238 is as follows:
- Uranium 238 (U-238) decays into thorium 234 (Th-234) through alpha decay, emitting an alpha particle (2 protons and 2 neutrons).
- Thorium 234 (Th-234) decays into protactinium 234 (Pa-234) through beta decay, emitting a beta particle (an electron).
- Protactinium 234 (Pa-234) decays into uranium 234 (U-234) through beta decay, emitting another beta particle.
The resulting thorium 234 is a radioactive element with an atomic number of 90 and an atomic mass of 234.
The Role of Uranium 238 and Thorium 234
Uranium 238 and thorium 234 have significant applications in various fields, including:
- Nuclear Energy: Uranium 238 is a key component of nuclear fuel, used to generate electricity in nuclear power plants. Thorium 234 is also a potential fuel source for nuclear reactors.
- Medicine: Thorium 234 is used in medical research, particularly in the development of cancer treatments and diagnostic tools.
- Scientific Research: Uranium 238 and thorium 234 are used in scientific research, including geology, physics, and chemistry.
Applications of Thorium 234
Thorium 234 has several applications in medicine, research, and industry, including:
- Cancer Treatment: Thorium 234 is used in cancer treatment, particularly in brachytherapy, where small radioactive sources are implanted in the body to kill cancer cells.
- Diagnostic Tools: Thorium 234 is used in diagnostic imaging, such as positron emission tomography (PET) scans.
- Nuclear Reactors: Thorium 234 is a potential fuel source for nuclear reactors, which could provide a safer and more efficient alternative to traditional nuclear fuels.

Benefits of Uranium 238 and Thorium 234
The decay of uranium 238 into thorium 234 has several benefits, including:
- Energy Generation: Uranium 238 and thorium 234 are used to generate electricity in nuclear power plants.
- Medical Applications: Thorium 234 is used in medical research and treatment, particularly in cancer therapy.
- Scientific Research: Uranium 238 and thorium 234 are used in scientific research, including geology, physics, and chemistry.
Challenges and Limitations
The decay of uranium 238 into thorium 234 also poses several challenges and limitations, including:
- Radioactive Waste: The decay of uranium 238 and thorium 234 produces radioactive waste, which requires careful management and disposal.
- Safety Concerns: The handling and storage of radioactive materials pose safety concerns for workers and the general public.
- Regulatory Framework: The use of uranium 238 and thorium 234 is regulated by strict laws and regulations, which can limit their applications.
Conclusion
The decay of uranium 238 into thorium 234 is a complex process that has significant implications for nuclear energy, medicine, and scientific research. Understanding the underlying mechanisms and applications of this process is crucial for harnessing the benefits of these radioactive elements. While there are challenges and limitations associated with the use of uranium 238 and thorium 234, ongoing research and development are addressing these concerns and exploring new applications for these elements.

Call to Action
We encourage readers to share their thoughts and questions about the decay of uranium 238 into thorium 234. How do you think this process can be harnessed for the benefit of society? What are the potential applications and challenges associated with the use of these radioactive elements? Join the conversation and let's explore the possibilities together!
FAQ Section
What is the half-life of uranium 238?
+The half-life of uranium 238 is approximately 4.5 billion years.
What is the primary application of thorium 234?
+The primary application of thorium 234 is in medical research and treatment, particularly in cancer therapy.
What are the challenges associated with the use of uranium 238 and thorium 234?
+The challenges associated with the use of uranium 238 and thorium 234 include radioactive waste management, safety concerns, and regulatory frameworks.