The world of chemistry is full of fascinating compounds, each with its unique properties and characteristics. One such compound that has garnered significant attention in recent years is fulminic acid. Also known as hydrogen cyanate, this compound has been a subject of interest for chemists and researchers due to its intriguing structure and potential applications. In this article, we will delve into the world of fulminic acid, exploring its major resonance form and other aspects of this captivating compound.
Fulminic acid is a colorless, volatile liquid with a pungent odor, consisting of hydrogen, carbon, nitrogen, and oxygen atoms. Its chemical formula is HCNO, and it is also known as hydrogen cyanate or isocyanic acid. This compound has been extensively studied due to its potential applications in various fields, including organic synthesis, materials science, and pharmaceuticals.
Understanding the Structure of Fulminic Acid

The structure of fulminic acid is characterized by the presence of a triple bond between the carbon and nitrogen atoms, making it a member of the nitrile family. This triple bond is responsible for the compound's high reactivity and its ability to participate in various chemical reactions. The hydrogen atom is bonded to the oxygen atom, forming a hydroxyl group, which plays a crucial role in the compound's acidity.
Major Resonance Form of Fulminic Acid
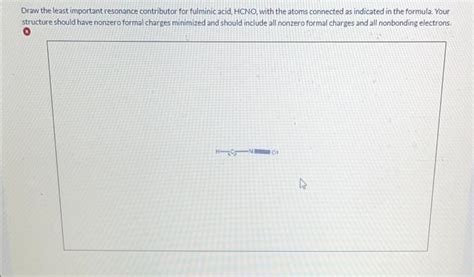
The major resonance form of fulminic acid is a topic of significant interest among chemists. Resonance forms are a way of representing the delocalization of electrons in a molecule, which is essential for understanding its chemical properties and behavior. The major resonance form of fulminic acid is depicted below:
HC≡N → O
In this resonance form, the electrons are delocalized between the carbon, nitrogen, and oxygen atoms, resulting in a partial double bond between the carbon and oxygen atoms. This delocalization of electrons is responsible for the compound's high reactivity and its ability to participate in various chemical reactions.
Properties and Applications of Fulminic Acid

Fulminic acid has several unique properties that make it an attractive compound for various applications. Some of its notable properties include:
- High reactivity: Fulminic acid is highly reactive due to the presence of the triple bond between the carbon and nitrogen atoms.
- Acidity: Fulminic acid is a strong acid, with a pKa value of around 3.5.
- Volatility: Fulminic acid is a volatile liquid, with a boiling point of around 23°C.
These properties make fulminic acid a useful compound in various applications, including:
- Organic synthesis: Fulminic acid is used as a reagent in various organic synthesis reactions, including the synthesis of heterocyclic compounds and pharmaceuticals.
- Materials science: Fulminic acid is used as a precursor to synthesize various materials, including nanoparticles and polymers.
- Pharmaceuticals: Fulminic acid is used as a building block to synthesize various pharmaceuticals, including antibacterial and antiviral agents.
Preparation and Synthesis of Fulminic Acid
Fulminic acid can be prepared and synthesized through various methods, including:
- Reaction of nitric acid with hydrogen cyanide
- Reaction of nitric acid with formic acid
- Reaction of nitric acid with urea
These methods involve the reaction of nitric acid with various reagents to produce fulminic acid. The resulting compound is then purified and isolated through various techniques, including distillation and crystallization.
Challenges and Future Directions

Despite its potential applications, fulminic acid poses several challenges, including:
- Instability: Fulminic acid is highly unstable and prone to decomposition, making it challenging to handle and store.
- Reactivity: Fulminic acid is highly reactive, which can make it difficult to work with and control.
To overcome these challenges, researchers are exploring new methods to synthesize and stabilize fulminic acid, including the use of novel catalysts and reaction conditions. Additionally, researchers are investigating new applications for fulminic acid, including its use in renewable energy and sustainable chemistry.
What is fulminic acid?
+Fulminic acid is a colorless, volatile liquid with a pungent odor, consisting of hydrogen, carbon, nitrogen, and oxygen atoms. Its chemical formula is HCNO, and it is also known as hydrogen cyanate or isocyanic acid.
What are the major resonance forms of fulminic acid?
+The major resonance form of fulminic acid is HC≡N → O, where the electrons are delocalized between the carbon, nitrogen, and oxygen atoms, resulting in a partial double bond between the carbon and oxygen atoms.
What are the applications of fulminic acid?
+Fulminic acid has several unique properties that make it an attractive compound for various applications, including organic synthesis, materials science, and pharmaceuticals.
In conclusion, fulminic acid is a fascinating compound with a rich history and a wide range of applications. Its unique properties, including its high reactivity and acidity, make it an attractive compound for various industries. However, its instability and reactivity also pose significant challenges, requiring careful handling and control. As researchers continue to explore new methods to synthesize and stabilize fulminic acid, its potential applications are likely to expand, making it an exciting compound to watch in the years to come.