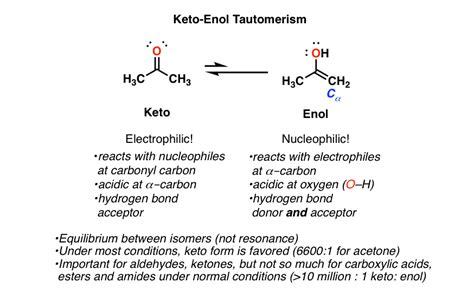
Keto vs Enol: Understanding the Basics

When it comes to organic chemistry, two important concepts that are often discussed are keto and enol. These terms refer to the structure and properties of certain organic compounds, particularly those containing carbonyl groups. In this article, we will delve into the world of keto and enol, exploring their differences and significance in the realm of organic chemistry.
At its core, the distinction between keto and enol lies in the arrangement of atoms within a molecule. This difference has a profound impact on the chemical and physical properties of the compound, influencing its reactivity, stability, and overall behavior. To grasp the fundamental principles of keto and enol, it is essential to understand the basic structure and bonding patterns involved.
The Keto Form: Structure and Properties

In the keto form, the carbonyl group (C=O) is the central feature. This group consists of a carbon atom double-bonded to an oxygen atom, resulting in a planar, trigonal geometry. The carbonyl group is highly polar, with the oxygen atom bearing a partial negative charge and the carbon atom carrying a partial positive charge. This polarity plays a crucial role in the chemical behavior of keto compounds.
The keto form is typically more stable than its enol counterpart, due to the greater delocalization of electrons within the molecule. This increased stability is reflected in the keto form's lower energy state, making it more resistant to chemical reactions.
Key Characteristics of Keto Compounds
• Polarity: Keto compounds exhibit a high degree of polarity, resulting from the carbonyl group's unequal sharing of electrons. • Reactivity: The keto form is generally less reactive than the enol form, due to its greater stability and lower energy state. • Stability: Keto compounds tend to be more stable than enol compounds, with a lower energy state and greater delocalization of electrons.
The Enol Form: Structure and Properties

In contrast to the keto form, the enol form features a hydroxyl group (OH) attached to a carbon atom that is also bonded to a carbonyl group. This arrangement results in a non-planar, tetrahedral geometry, with the hydroxyl group positioned perpendicular to the plane of the carbonyl group.
The enol form is typically less stable than the keto form, due to the reduced delocalization of electrons within the molecule. This decreased stability is reflected in the enol form's higher energy state, making it more reactive and susceptible to chemical transformations.
Key Characteristics of Enol Compounds
• Polarity: Enol compounds exhibit a lower degree of polarity compared to keto compounds, due to the reduced influence of the carbonyl group. • Reactivity: The enol form is generally more reactive than the keto form, due to its higher energy state and reduced stability. • Stability: Enol compounds tend to be less stable than keto compounds, with a higher energy state and reduced delocalization of electrons.
Keto-Enol Tautomerism: The Equilibrium Between Forms

In many cases, keto and enol compounds can interconvert through a process known as keto-enol tautomerism. This equilibrium involves the transfer of a proton (H+ ion) between the keto and enol forms, resulting in a mixture of both forms in solution.
The keto-enol equilibrium is influenced by various factors, including the solvent, temperature, and presence of catalysts. Understanding this equilibrium is crucial in predicting the behavior of keto and enol compounds in different chemical environments.
Factors Influencing Keto-Enol Tautomerism
• Solvent: The choice of solvent can significantly impact the keto-enol equilibrium, with some solvents favoring the keto form and others favoring the enol form. • Temperature: Changes in temperature can influence the equilibrium, with higher temperatures often favoring the keto form. • Catalysts: The presence of catalysts, such as acids or bases, can accelerate the keto-enol interconversion and alter the equilibrium.
Practical Applications of Keto and Enol Compounds

Keto and enol compounds have numerous practical applications in various fields, including:
• Pharmaceuticals: Many pharmaceutical compounds, such as pain relievers and antibiotics, contain keto or enol groups. • Agricultural chemicals: Pesticides and herbicides often rely on keto or enol compounds for their efficacy. • Materials science: Keto and enol compounds are used in the development of advanced materials, such as polymers and coatings.
Conclusion
In conclusion, the distinction between keto and enol forms is a fundamental concept in organic chemistry, with significant implications for the structure, properties, and reactivity of compounds. Understanding the differences between keto and enol compounds is crucial in predicting their behavior and harnessing their potential in various applications.
By grasping the principles of keto and enol chemistry, researchers and scientists can develop new compounds and materials with unique properties, leading to advancements in fields such as medicine, agriculture, and materials science.
What is the main difference between keto and enol forms?
+The main difference between keto and enol forms lies in the arrangement of atoms within the molecule. The keto form features a carbonyl group (C=O), while the enol form features a hydroxyl group (OH) attached to a carbon atom that is also bonded to a carbonyl group.
Which form is generally more stable, keto or enol?
+The keto form is typically more stable than the enol form, due to the greater delocalization of electrons within the molecule.
What is keto-enol tautomerism, and how does it affect the equilibrium between forms?
+Keto-enol tautomerism is the process by which keto and enol compounds interconvert through the transfer of a proton (H+ ion). This equilibrium is influenced by factors such as the solvent, temperature, and presence of catalysts.