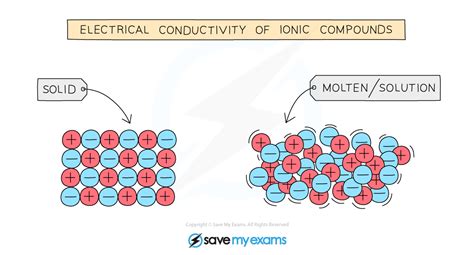
The ability of ionic compounds to conduct electricity is a fundamental concept in chemistry and physics. Ionic compounds, which are formed when one or more electrons are transferred between atoms, can conduct electricity in various ways. In this article, we will explore four ways ionic compounds conduct electricity, including the movement of ions, the formation of electrolytes, the creation of ionic crystals, and the use of molten salts.

Movement of Ions
Ionic compounds conduct electricity through the movement of ions. When an ionic compound is dissolved in water, the ions are free to move. The positively charged ions, known as cations, move towards the negatively charged electrode, while the negatively charged ions, known as anions, move towards the positively charged electrode. This movement of ions allows the compound to conduct electricity.
For example, when sodium chloride (NaCl) is dissolved in water, the sodium ions (Na+) move towards the negatively charged electrode, while the chloride ions (Cl-) move towards the positively charged electrode. This movement of ions allows the compound to conduct electricity.
Formation of Electrolytes
Ionic compounds can also conduct electricity through the formation of electrolytes. An electrolyte is a substance that conducts electricity when dissolved in water. When an ionic compound is dissolved in water, it breaks down into its constituent ions, which are then free to move and conduct electricity.
For example, when sulfuric acid (H2SO4) is dissolved in water, it breaks down into hydrogen ions (H+) and sulfate ions (SO42-). The movement of these ions allows the compound to conduct electricity.

Creation of Ionic Crystals
Ionic compounds can also conduct electricity through the creation of ionic crystals. Ionic crystals are formed when ions are arranged in a repeating pattern, known as a crystal lattice. The ions in the crystal lattice are free to move, allowing the compound to conduct electricity.
For example, when sodium chloride (NaCl) is heated, it forms an ionic crystal. The sodium ions and chloride ions in the crystal lattice are free to move, allowing the compound to conduct electricity.
Use of Molten Salts
Ionic compounds can also conduct electricity through the use of molten salts. Molten salts are formed when ionic compounds are heated to high temperatures, causing the ions to become free to move. The movement of these ions allows the compound to conduct electricity.
For example, when sodium nitrate (NaNO3) is heated to high temperatures, it forms a molten salt. The sodium ions and nitrate ions in the molten salt are free to move, allowing the compound to conduct electricity.

Practical Applications
The ability of ionic compounds to conduct electricity has many practical applications. For example, ionic compounds are used in batteries, which rely on the movement of ions to generate electricity. Ionic compounds are also used in electroplating, which involves the deposition of ions onto a surface to create a thin layer of metal.
In addition, ionic compounds are used in the production of aluminum, which involves the electrolysis of molten salt. The movement of ions in the molten salt allows the aluminum to be deposited at the cathode.
Conclusion
In conclusion, ionic compounds conduct electricity through the movement of ions, the formation of electrolytes, the creation of ionic crystals, and the use of molten salts. The ability of ionic compounds to conduct electricity has many practical applications, including the use in batteries, electroplating, and the production of aluminum. We hope this article has provided a comprehensive understanding of the ways in which ionic compounds conduct electricity.
We encourage you to share your thoughts and questions in the comments below. Have you worked with ionic compounds before? What are some of the practical applications that you have encountered?
What is an ionic compound?
+An ionic compound is a compound that is formed when one or more electrons are transferred between atoms. This transfer of electrons results in the formation of ions, which are atoms that have gained or lost electrons.
How do ionic compounds conduct electricity?
+Ionic compounds conduct electricity through the movement of ions. When an ionic compound is dissolved in water, the ions are free to move, allowing the compound to conduct electricity.
What are some practical applications of ionic compounds?
+Ionic compounds have many practical applications, including the use in batteries, electroplating, and the production of aluminum.