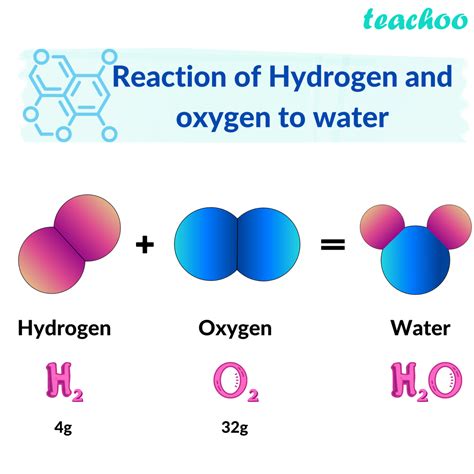
The simple yet profound combination of hydrogen and oxygen forming water is one of the most fundamental concepts in chemistry. Water, a compound made up of two hydrogen atoms and one oxygen atom (H2O), is essential for human life and plays a critical role in many biological and chemical processes. In this article, we will delve into the world of hydrogen and oxygen, exploring their individual properties, the process of combining them to form water, and the significance of this compound in our daily lives.
Hydrogen: The Lightest and Most Abundant Element

Hydrogen is the lightest and most abundant element in the universe, making up approximately 75% of its elemental mass. It is a highly reactive gas, which readily forms compounds with other elements. Hydrogen has a single electron in its outermost shell, making it eager to form bonds with other atoms to achieve a more stable configuration. This reactivity is the key to its ability to combine with oxygen to form water.
Properties of Hydrogen
- Highly flammable and explosive when combined with air
- Can exist in three main forms: atomic hydrogen (H), molecular hydrogen (H2), and hydrogen ions (H+)
- Highly reactive and readily forms compounds with other elements
- Essential for life and a key component of many biological molecules, such as proteins and carbohydrates
Oxygen: The Breath of Life

Oxygen, on the other hand, is a vital element that makes up approximately 21% of the Earth's atmosphere. It is essential for human life, as we need oxygen to breathe and produce energy. Oxygen is a highly reactive element, particularly in its molecular form (O2), where two oxygen atoms share a covalent bond. This reactivity allows oxygen to readily form compounds with other elements, including hydrogen.
Properties of Oxygen
- Essential for human life and energy production
- Highly reactive, particularly in its molecular form (O2)
- Exists in several forms, including molecular oxygen (O2), atomic oxygen (O), and oxygen ions (O2-)
- Crucial for many biological processes, including respiration and photosynthesis
The Formation of Water: A Chemical Reaction

When hydrogen and oxygen combine, they form a chemical bond that results in the creation of water (H2O). This reaction is highly exothermic, releasing energy in the form of heat. The equation for this reaction is:
2H2 + O2 → 2H2O
In this reaction, two molecules of hydrogen gas (H2) combine with one molecule of oxygen gas (O2) to form two molecules of water (H2O). This reaction is a fundamental process that occurs naturally in many environments, including the human body.
Steps Involved in the Formation of Water
- Hydrogen and oxygen molecules collide, resulting in the sharing of electrons and the formation of a covalent bond.
- The two hydrogen atoms bond with the oxygen atom, resulting in the formation of a water molecule (H2O).
- Energy is released in the form of heat, making the reaction highly exothermic.
Significance of Water in Our Daily Lives

Water is essential for human life and plays a critical role in many biological and chemical processes. It is a universal solvent, capable of dissolving a wide range of substances, and is necessary for many industrial and agricultural applications. Some of the significance of water in our daily lives includes:
- Human consumption: Water is essential for human survival, and we need it to drink, cook, and maintain personal hygiene.
- Agriculture: Water is necessary for crop growth and irrigation, making it a critical component of food production.
- Industry: Water is used in many industrial processes, including manufacturing, energy production, and transportation.
Importance of Water in Biological Processes
- Cell growth and division: Water is necessary for cell growth and division, making it essential for the development and maintenance of tissues.
- Temperature regulation: Water helps regulate body temperature, making it essential for maintaining homeostasis.
- Waste removal: Water helps remove waste products from the body, making it essential for maintaining proper bodily functions.
Conclusion and Final Thoughts
In conclusion, the combination of hydrogen and oxygen to form water is a fundamental process that is essential for human life and many biological and chemical processes. Understanding the properties of these elements and the reaction that forms water is crucial for appreciating the significance of this compound in our daily lives. We hope that this article has provided you with a deeper understanding of the importance of water and the chemical reaction that forms it.
What is the chemical equation for the formation of water?
+The chemical equation for the formation of water is 2H2 + O2 → 2H2O.
What are the properties of hydrogen?
+Hydrogen is highly flammable and explosive, highly reactive, and essential for life.
What is the significance of water in our daily lives?
+Water is essential for human consumption, agriculture, industry, and many biological processes.