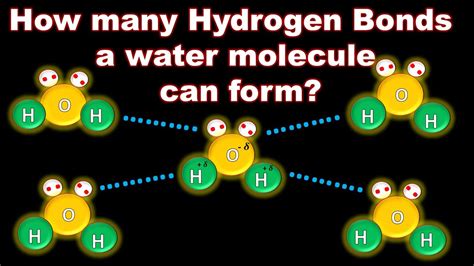
The fascinating world of chemistry! Hydrogen bonds play a crucial role in the structure and properties of water, making it an essential component of life on Earth. But have you ever wondered how many hydrogen bonds a single water molecule can form?

Understanding Hydrogen Bonds
Hydrogen bonds are a type of intermolecular force that arises between molecules with a hydrogen atom bonded to a highly electronegative atom, such as oxygen, nitrogen, or fluorine. In the case of water, hydrogen bonds form between the hydrogen atoms of one water molecule and the oxygen atom of another. These bonds are relatively weak compared to covalent bonds, but they play a significant role in determining the physical and chemical properties of water.
The Structure of a Water Molecule
A water molecule (H2O) consists of two hydrogen atoms bonded to a single oxygen atom. The oxygen atom has a slightly negative charge due to its high electronegativity, while the hydrogen atoms have a slightly positive charge. This creates a partial positive charge on the hydrogen atoms and a partial negative charge on the oxygen atom.

Hydrogen Bonding in Water
When two water molecules come into close proximity, the partially positive hydrogen atoms of one molecule are attracted to the partially negative oxygen atom of another molecule. This attraction leads to the formation of a hydrogen bond. In the case of water, each hydrogen atom can form a hydrogen bond with an oxygen atom of another water molecule.
How Many Hydrogen Bonds Can a Water Molecule Form?
A single water molecule can form a maximum of four hydrogen bonds with neighboring water molecules. Two of these bonds are formed by the hydrogen atoms of the water molecule, and the other two are formed by the oxygen atom.
- The two hydrogen atoms of a water molecule can each form a hydrogen bond with an oxygen atom of another water molecule.
- The oxygen atom of a water molecule can form two hydrogen bonds with the hydrogen atoms of two other water molecules.

Importance of Hydrogen Bonds in Water
The ability of a water molecule to form four hydrogen bonds is crucial for its unique properties and behavior. Hydrogen bonds are responsible for:
- The high boiling point of water: Hydrogen bonds require a significant amount of energy to break, which is why water boils at a relatively high temperature (100°C).
- The surface tension of water: Hydrogen bonds between water molecules at the surface create a "skin" that allows water to resist external forces and maintain its shape against gravity.
- The viscosity of water: Hydrogen bonds between water molecules affect the flow of water, making it more viscous than other liquids.

Conclusion
In conclusion, a single water molecule can form four hydrogen bonds with neighboring water molecules. These bonds are essential for the unique properties and behavior of water, making it an essential component of life on Earth.
Now, take a moment to appreciate the intricate world of chemistry and the fascinating properties of water!
What is a hydrogen bond?
+A hydrogen bond is a type of intermolecular force that arises between molecules with a hydrogen atom bonded to a highly electronegative atom.
How many hydrogen bonds can a water molecule form?
+A single water molecule can form a maximum of four hydrogen bonds with neighboring water molecules.
What is the importance of hydrogen bonds in water?
+Hydrogen bonds are responsible for the high boiling point, surface tension, and viscosity of water, making it an essential component of life on Earth.