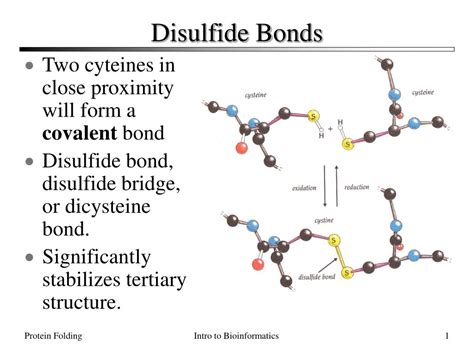
Disulfide bonds are a crucial aspect of protein structure and function, playing a vital role in maintaining the stability and integrity of proteins. These bonds are formed between two cysteine residues, which are amino acids that contain a thiol (-SH) group. The formation of disulfide bonds is a complex process that involves several steps and mechanisms. In this article, we will explore five ways disulfide bonds form and their significance in protein biology.
What are Disulfide Bonds?

Disulfide bonds, also known as disulfide bridges or S-S bonds, are covalent bonds formed between two cysteine residues in a protein. These bonds are crucial for maintaining the native conformation of proteins, which is essential for their function and stability. Disulfide bonds are formed through a process called oxidation, where the thiol groups of two cysteine residues are oxidized to form a sulfur-sulfur bond.
1. Oxidation of Thiol Groups

One way disulfide bonds form is through the oxidation of thiol groups. This process involves the conversion of the thiol groups of two cysteine residues into a sulfur-sulfur bond. The oxidation reaction is catalyzed by enzymes called oxidoreductases, which transfer electrons from the thiol groups to an electron acceptor. This process results in the formation of a disulfide bond between the two cysteine residues.
Role of Oxidoreductases
Oxidoreductases play a crucial role in the formation of disulfide bonds. These enzymes catalyze the oxidation reaction by transferring electrons from the thiol groups to an electron acceptor. The most common oxidoreductase involved in disulfide bond formation is protein disulfide isomerase (PDI). PDI is a multifunctional enzyme that catalyzes the formation and rearrangement of disulfide bonds in proteins.
2. Disulfide Bond Formation through Thiol-Disulfide Exchange

Another way disulfide bonds form is through thiol-disulfide exchange. This process involves the exchange of thiol groups between two cysteine residues, resulting in the formation of a disulfide bond. Thiol-disulfide exchange is a reversible reaction that is catalyzed by enzymes such as PDI.
Role of Glutathione
Glutathione is a tripeptide that plays a crucial role in thiol-disulfide exchange. Glutathione is a reducing agent that can donate electrons to thiol groups, resulting in the formation of a disulfide bond. Glutathione is also involved in the reduction of disulfide bonds, making it a key player in the regulation of disulfide bond formation.
3. Disulfide Bond Formation through Enzyme-Catalyzed Reactions

Disulfide bonds can also form through enzyme-catalyzed reactions. These reactions involve the catalysis of disulfide bond formation by specific enzymes. One example of an enzyme-catalyzed reaction is the formation of disulfide bonds in proteins through the action of sulfhydryl oxidases.
Role of Sulfhydryl Oxidases
Sulfhydryl oxidases are enzymes that catalyze the formation of disulfide bonds in proteins. These enzymes transfer electrons from thiol groups to an electron acceptor, resulting in the formation of a disulfide bond. Sulfhydryl oxidases are involved in the formation of disulfide bonds in a variety of proteins, including those involved in protein folding and stability.
4. Disulfide Bond Formation through Chemical Oxidation

Disulfide bonds can also form through chemical oxidation. This process involves the oxidation of thiol groups by chemical reagents, resulting in the formation of a disulfide bond. Chemical oxidation is commonly used in the laboratory to form disulfide bonds in proteins.
Role of Oxidizing Agents
Oxidizing agents, such as hydrogen peroxide, are commonly used to form disulfide bonds through chemical oxidation. These agents transfer electrons from thiol groups to an electron acceptor, resulting in the formation of a disulfide bond. Oxidizing agents are widely used in the laboratory to study the structure and function of proteins.
5. Disulfide Bond Formation through Redox Reactions

Disulfide bonds can also form through redox reactions. These reactions involve the transfer of electrons from thiol groups to an electron acceptor, resulting in the formation of a disulfide bond. Redox reactions are commonly used in the laboratory to study the structure and function of proteins.
Role of Redox Enzymes
Redox enzymes, such as thioredoxin, play a crucial role in the formation of disulfide bonds through redox reactions. These enzymes transfer electrons from thiol groups to an electron acceptor, resulting in the formation of a disulfide bond. Redox enzymes are involved in the regulation of disulfide bond formation in a variety of proteins.
In conclusion, disulfide bonds form through a variety of mechanisms, including oxidation of thiol groups, thiol-disulfide exchange, enzyme-catalyzed reactions, chemical oxidation, and redox reactions. These mechanisms involve the transfer of electrons from thiol groups to an electron acceptor, resulting in the formation of a disulfide bond. Understanding the mechanisms of disulfide bond formation is crucial for understanding the structure and function of proteins.
What are disulfide bonds?
+Disulfide bonds are covalent bonds formed between two cysteine residues in a protein.
What is the role of oxidoreductases in disulfide bond formation?
+Oxidoreductases catalyze the oxidation reaction by transferring electrons from thiol groups to an electron acceptor.
What is the role of glutathione in thiol-disulfide exchange?
+Glutathione is a reducing agent that can donate electrons to thiol groups, resulting in the formation of a disulfide bond.