In the world of chemistry, bonds are the connections between atoms that hold molecules together. While single bonds are the most common type of bond, double and triple bonds play a crucial role in the structure and function of many molecules. But have you ever wondered how these multiple bonds form?
In this article, we'll explore the three main ways double and triple bonds form, along with their mechanisms and examples. Whether you're a chemistry enthusiast or a student looking to deepen your understanding of molecular bonding, this article is for you.

What are Double and Triple Bonds?
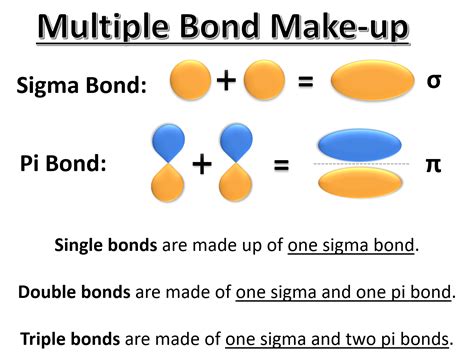
Before we dive into how double and triple bonds form, let's quickly review what they are. A double bond is a type of covalent bond that involves the sharing of four electrons between two atoms, while a triple bond involves the sharing of six electrons. These multiple bonds are stronger and shorter than single bonds, which is why they're often found in molecules with unique properties.
Double Bonds
Double bonds are characterized by a sigma (σ) bond and a pi (π) bond. The σ bond is formed by the end-to-end overlap of atomic orbitals, while the π bond is formed by the side-by-side overlap of parallel orbitals. Double bonds are commonly found in molecules like alkenes, alkynes, and aromatics.
Triple Bonds
Triple bonds, on the other hand, are characterized by a sigma (σ) bond and two pi (π) bonds. Like double bonds, triple bonds are formed by the overlap of atomic orbitals, but with an additional π bond. Triple bonds are commonly found in molecules like alkynes and nitriles.
1. Sigma-Pi (σ-π) Bonding Mechanism
The first way double and triple bonds form is through the sigma-pi (σ-π) bonding mechanism. This mechanism involves the overlap of atomic orbitals to form a σ bond, followed by the overlap of parallel orbitals to form one or more π bonds.

In the case of double bonds, the σ bond is formed by the overlap of two sp2 hybrid orbitals, while the π bond is formed by the overlap of two parallel p orbitals. For triple bonds, the σ bond is formed by the overlap of two sp hybrid orbitals, while the two π bonds are formed by the overlap of two parallel p orbitals.
2. pi-pi (π-π) Bonding Mechanism
The second way double and triple bonds form is through the pi-pi (π-π) bonding mechanism. This mechanism involves the overlap of parallel orbitals to form one or more π bonds.
pi-pi Bonding in Double Bonds
In the case of double bonds, the π-π bonding mechanism involves the overlap of two parallel p orbitals to form a π bond. This mechanism is commonly found in molecules like alkenes and alkynes.

pi-pi Bonding in Triple Bonds
For triple bonds, the π-π bonding mechanism involves the overlap of two parallel p orbitals to form two π bonds. This mechanism is commonly found in molecules like alkynes and nitriles.
3. sp Hybridization Mechanism
The third way double and triple bonds form is through the sp hybridization mechanism. This mechanism involves the hybridization of atomic orbitals to form sp hybrid orbitals, which then overlap to form a σ bond.

In the case of double bonds, the sp hybridization mechanism involves the hybridization of one s and one p orbital to form two sp2 hybrid orbitals, which then overlap to form a σ bond. For triple bonds, the sp hybridization mechanism involves the hybridization of one s and one p orbital to form two sp hybrid orbitals, which then overlap to form a σ bond.
Examples and Applications
Double and triple bonds are found in a wide range of molecules, from simple alkenes and alkynes to complex biomolecules like proteins and DNA.
- Alkenes, like ethene (C2H4), are used as monomers in the production of polyethylene plastics.
- Alkynes, like acetylene (C2H2), are used as fuels and in the production of various chemicals.
- Aromatics, like benzene (C6H6), are used as solvents and in the production of various chemicals.
- Proteins, like collagen, contain triple bonds that provide structural strength and elasticity.
- DNA, the molecule of life, contains double bonds that play a crucial role in its structure and function.
Conclusion: The Importance of Double and Triple Bonds
Double and triple bonds are essential components of many molecules, from simple organic compounds to complex biomolecules. Understanding how these multiple bonds form is crucial for understanding the structure and function of these molecules.
By exploring the three main ways double and triple bonds form – sigma-pi (σ-π) bonding mechanism, pi-pi (π-π) bonding mechanism, and sp hybridization mechanism – we can gain a deeper appreciation for the complex world of molecular bonding.

Whether you're a chemistry enthusiast or a student looking to deepen your understanding of molecular bonding, we hope this article has provided you with a comprehensive and engaging overview of double and triple bonds.
So, what do you think? Do you have any questions or topics you'd like to discuss? Share your thoughts in the comments below!
What is the difference between a double bond and a triple bond?
+A double bond is a type of covalent bond that involves the sharing of four electrons between two atoms, while a triple bond involves the sharing of six electrons. Double bonds are characterized by a sigma (σ) bond and a pi (π) bond, while triple bonds are characterized by a sigma (σ) bond and two pi (π) bonds.
What is the sigma-pi (σ-π) bonding mechanism?
+The sigma-pi (σ-π) bonding mechanism involves the overlap of atomic orbitals to form a σ bond, followed by the overlap of parallel orbitals to form one or more π bonds. This mechanism is commonly found in molecules like alkenes and alkynes.
What is the importance of double and triple bonds in biomolecules?
+Double and triple bonds play a crucial role in the structure and function of biomolecules like proteins and DNA. They provide structural strength and elasticity, and are involved in various biochemical reactions.