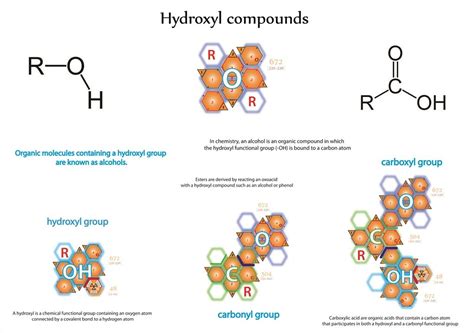
Hydrogen bonds are a crucial aspect of chemistry and biology, playing a vital role in the structure and function of molecules. Hydroxyl groups, which consist of a hydrogen atom bonded to an oxygen atom, are one of the most common groups involved in forming hydrogen bonds. In this article, we will delve into the world of hydrogen bonding and explore five ways hydroxyl groups form these essential interactions.
The Importance of Hydrogen Bonds

Hydrogen bonds are weak electrostatic attractions between a hydrogen atom bonded to a highly electronegative atom, such as oxygen, nitrogen, or fluorine, and another electronegative atom. These bonds are responsible for the structure and properties of many biological molecules, including DNA, proteins, and water. Hydrogen bonds also play a crucial role in the solubility and boiling points of substances.
What are Hydroxyl Groups?

Hydroxyl groups, also known as hydroxy groups or hydroxides, consist of a hydrogen atom bonded to an oxygen atom. This functional group is commonly found in organic compounds, such as alcohols, carbohydrates, and amino acids. Hydroxyl groups are highly polar, meaning they have a slightly positive charge on the hydrogen atom and a slightly negative charge on the oxygen atom. This polarity allows hydroxyl groups to form hydrogen bonds with other electronegative atoms.
1. Hydrogen Bonding between Hydroxyl Groups
One of the most common ways hydroxyl groups form hydrogen bonds is by interacting with other hydroxyl groups. This type of hydrogen bonding is known as intermolecular hydrogen bonding. When two hydroxyl groups are in close proximity, the slightly positive hydrogen atom of one group is attracted to the slightly negative oxygen atom of another group. This attraction creates a weak electrostatic bond between the two molecules.
Hydrogen Bonding in Water

Water is a unique substance that exhibits strong hydrogen bonding between its molecules. Each water molecule has two hydroxyl groups, which are able to form hydrogen bonds with other water molecules. This extensive network of hydrogen bonds gives water its high boiling point, surface tension, and viscosity.
2. Hydrogen Bonding between Hydroxyl Groups and Other Electronegative Atoms
Hydroxyl groups can also form hydrogen bonds with other electronegative atoms, such as nitrogen, fluorine, and chlorine. This type of hydrogen bonding is known as intermolecular hydrogen bonding. When a hydroxyl group is in close proximity to another electronegative atom, the slightly positive hydrogen atom is attracted to the slightly negative atom. This attraction creates a weak electrostatic bond between the two molecules.
Hydrogen Bonding in Biological Molecules

Hydrogen bonding plays a crucial role in the structure and function of biological molecules, including DNA, proteins, and carbohydrates. Hydroxyl groups are commonly found in these molecules and are able to form hydrogen bonds with other electronegative atoms. These hydrogen bonds help to stabilize the structure of the molecule and facilitate its function.
3. Hydrogen Bonding between Hydroxyl Groups and Metal Ions
Hydroxyl groups can also form hydrogen bonds with metal ions, such as sodium, potassium, and calcium. This type of hydrogen bonding is known as ion-dipole hydrogen bonding. When a hydroxyl group is in close proximity to a metal ion, the slightly positive hydrogen atom is attracted to the slightly negative ion. This attraction creates a weak electrostatic bond between the two molecules.
Hydrogen Bonding in Materials Science

Hydrogen bonding plays a crucial role in the development of new materials, including polymers, ceramics, and nanomaterials. Hydroxyl groups are commonly used to functionalize the surface of materials, allowing them to form hydrogen bonds with other molecules. This can improve the strength, durability, and functionality of the material.
4. Hydrogen Bonding between Hydroxyl Groups and π-Systems
Hydroxyl groups can also form hydrogen bonds with π-systems, such as benzene rings and double bonds. This type of hydrogen bonding is known as π-hydrogen bonding. When a hydroxyl group is in close proximity to a π-system, the slightly positive hydrogen atom is attracted to the slightly negative π-electrons. This attraction creates a weak electrostatic bond between the two molecules.
Hydrogen Bonding in Catalysis

Hydrogen bonding plays a crucial role in catalysis, allowing catalysts to facilitate chemical reactions. Hydroxyl groups are commonly used to functionalize the surface of catalysts, allowing them to form hydrogen bonds with reactant molecules. This can improve the efficiency and selectivity of the reaction.
5. Hydrogen Bonding between Hydroxyl Groups and Hydrogen Fluoride
Hydroxyl groups can also form hydrogen bonds with hydrogen fluoride, a highly electronegative molecule. This type of hydrogen bonding is known as F-H-O hydrogen bonding. When a hydroxyl group is in close proximity to hydrogen fluoride, the slightly positive hydrogen atom is attracted to the slightly negative fluorine atom. This attraction creates a weak electrostatic bond between the two molecules.
Now that we've explored the five ways hydroxyl groups form hydrogen bonds, it's clear that these interactions play a vital role in many areas of chemistry and biology. By understanding how hydroxyl groups form hydrogen bonds, we can better appreciate the complex structures and functions of molecules and develop new materials and technologies.
We invite you to share your thoughts on the importance of hydrogen bonding and hydroxyl groups in the comments section below. How do you think hydrogen bonding affects the properties of molecules and materials? Share your insights and let's start a conversation!
What is a hydroxyl group?
+A hydroxyl group is a functional group consisting of a hydrogen atom bonded to an oxygen atom.
What is hydrogen bonding?
+Hydrogen bonding is a weak electrostatic attraction between a hydrogen atom bonded to a highly electronegative atom and another electronegative atom.
Why is hydrogen bonding important?
+Hydrogen bonding plays a crucial role in the structure and function of molecules, including DNA, proteins, and water.