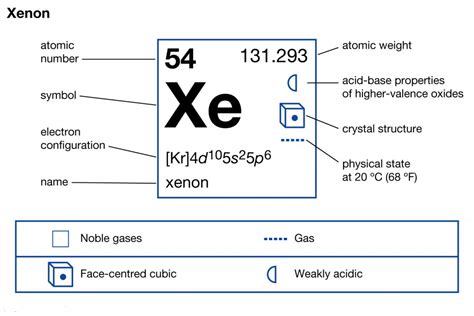
Xenon, a noble gas, is often misunderstood due to its seemingly complex electron configuration. However, by breaking it down into simple terms, we can easily grasp the concept. In this article, we will delve into the world of Xenon's electron configuration, exploring its significance, benefits, and practical applications.
Xenon's electron configuration is a fundamental concept in chemistry, essential for understanding the properties and behavior of this noble gas. By gaining a deeper understanding of Xenon's electron configuration, we can unlock its potential and harness its unique characteristics. In this article, we will take a step-by-step approach to explaining Xenon's electron configuration, making it accessible to readers of all levels.
Xenon Electron Configuration Basics

To comprehend Xenon's electron configuration, we first need to understand the basic principles of electron configuration. Electron configuration refers to the arrangement of electrons within an atom, which determines its chemical properties and behavior. The electron configuration of an atom is typically written in a specific notation, which provides information about the energy levels, orbitals, and number of electrons.
Xenon's atomic number is 54, which means it has 54 protons and 54 electrons. The electron configuration of Xenon can be written as:
1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁶ 4d¹⁰ 5s² 5p⁶
This notation may seem daunting, but by breaking it down, we can understand the arrangement of Xenon's electrons.
Xenon's Energy Levels
Xenon's electron configuration consists of multiple energy levels, each with a specific number of electrons. The energy levels are labeled as 1s, 2s, 2p, 3s, 3p, 3d, 4s, 4p, 4d, 5s, and 5p. Each energy level has a unique capacity, and the electrons occupy these levels in a specific order.- The 1s energy level can hold up to 2 electrons.
- The 2s energy level can hold up to 2 electrons.
- The 2p energy level can hold up to 6 electrons.
- The 3s energy level can hold up to 2 electrons.
- The 3p energy level can hold up to 6 electrons.
- The 3d energy level can hold up to 10 electrons.
- The 4s energy level can hold up to 2 electrons.
- The 4p energy level can hold up to 6 electrons.
- The 4d energy level can hold up to 10 electrons.
- The 5s energy level can hold up to 2 electrons.
- The 5p energy level can hold up to 6 electrons.
By understanding the energy levels and their capacities, we can visualize the arrangement of Xenon's electrons.
Xenon's Electron Configuration Notation

Xenon's electron configuration notation is a shorthand way of representing the arrangement of its electrons. The notation consists of the energy level label (e.g., 1s, 2s, 2p) followed by a superscript number indicating the number of electrons in that energy level.
For example, the notation "1s²" indicates that the 1s energy level has 2 electrons. Similarly, "2p⁶" indicates that the 2p energy level has 6 electrons.
By understanding the notation, we can quickly identify the arrangement of Xenon's electrons.
Xenon's Electron Configuration Diagram
A diagram can help visualize Xenon's electron configuration. The diagram consists of a series of energy levels, with each level represented by a horizontal line. The electrons are represented by arrows, which indicate the spin of the electrons.Here is a simplified diagram of Xenon's electron configuration:
1s ↑↓
2s ↑↓
2p ↑↓↑↓↑↓
3s ↑↓
3p ↑↓↑↓↑↓
3d ↑↓↑↓↑↓↑↓↑↓
4s ↑↓
4p ↑↓↑↓↑↓
4d ↑↓↑↓↑↓↑↓↑↓
5s ↑↓
5p ↑↓↑↓↑↓
This diagram shows the arrangement of Xenon's electrons, with each energy level filled to its maximum capacity.
Practical Applications of Xenon's Electron Configuration

Xenon's electron configuration has several practical applications. Some of the most notable include:
- Lighting: Xenon is used in high-intensity lamps, such as xenon headlights and strobe lights.
- Lasers: Xenon is used in excimer lasers, which are used in eye surgery and semiconductor manufacturing.
- Anesthesia: Xenon is used as a general anesthetic, due to its ability to bind to proteins in the brain.
- Space Exploration: Xenon is used as a propellant in ion thrusters, which are used in spacecraft.
By understanding Xenon's electron configuration, we can unlock its potential and harness its unique characteristics.
Benefits of Xenon's Electron Configuration
Xenon's electron configuration has several benefits, including:- High ionization energy: Xenon's high ionization energy makes it an ideal gas for use in high-intensity lamps.
- Low reactivity: Xenon's low reactivity makes it an ideal gas for use in lasers and anesthesia.
- High thermal conductivity: Xenon's high thermal conductivity makes it an ideal gas for use in heat transfer applications.
By understanding the benefits of Xenon's electron configuration, we can appreciate its unique characteristics and potential applications.
Conclusion
In conclusion, Xenon's electron configuration is a complex yet fascinating topic. By breaking it down into simple terms, we can understand the arrangement of its electrons and unlock its potential. With its unique characteristics and practical applications, Xenon's electron configuration is an essential concept in chemistry and physics.We hope this article has helped you understand Xenon's electron configuration and its significance. If you have any questions or comments, please feel free to share them below.
What is the atomic number of Xenon?
+The atomic number of Xenon is 54.
What is the electron configuration of Xenon?
+The electron configuration of Xenon is 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁶ 4d¹⁰ 5s² 5p⁶.
What are some practical applications of Xenon's electron configuration?
+Xenon's electron configuration has several practical applications, including lighting, lasers, anesthesia, and space exploration.