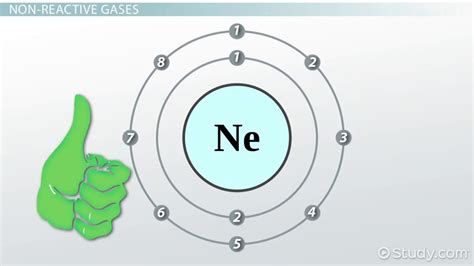
The noble gases, also known as the inert gases, are a group of elements in the periodic table that are known for their unreactive nature. They are located in the far right column of the periodic table and include elements such as helium, neon, argon, krypton, xenon, and radon. These elements are unique in that they have a full outer energy level, which makes them extremely stable and resistant to forming bonds with other elements.
The noble gases have been a subject of interest for scientists for many years, and their unique properties have led to many important applications in fields such as lighting, lasers, and medicine. But what makes these elements so special, and why do they refuse to form bonds with other elements?
Electron Configuration: The Key to the Noble Gases' Stability

The noble gases' stability can be attributed to their electron configuration. The electron configuration of an atom is the arrangement of electrons in its outer energy level. The noble gases have a full outer energy level, which means that their outermost energy level is completely filled with electrons. This full outer energy level makes it difficult for the noble gases to form bonds with other elements.
In general, atoms tend to gain, lose, or share electrons to achieve a full outer energy level. This is known as the octet rule, which states that atoms tend to gain, lose, or share electrons to achieve a full outer energy level with eight electrons. The noble gases, however, already have a full outer energy level, so they do not need to gain, lose, or share electrons to achieve stability.
The Role of Electron Shells in the Noble Gases' Stability

The electron shells of the noble gases also play a crucial role in their stability. The electron shells are the regions around the nucleus of an atom where the electrons are found. The noble gases have a full outer energy level, which means that their outermost electron shell is completely filled with electrons.
The full outer energy level of the noble gases makes it difficult for them to form bonds with other elements. When two atoms form a bond, they share or exchange electrons to achieve a full outer energy level. However, the noble gases already have a full outer energy level, so they do not need to share or exchange electrons to achieve stability.
Factors That Contribute to the Noble Gases' Stability
Several factors contribute to the noble gases' stability, including:
- High ionization energy: The noble gases have high ionization energies, which makes it difficult for them to lose electrons and form bonds with other elements.
- Low electron affinity: The noble gases have low electron affinities, which makes it difficult for them to gain electrons and form bonds with other elements.
- Full outer energy level: The noble gases have a full outer energy level, which makes it difficult for them to share or exchange electrons to achieve stability.
Applications of the Noble Gases

Despite their unreactive nature, the noble gases have many important applications in various fields. Some of the applications of the noble gases include:
- Lighting: The noble gases are used in lighting, such as neon signs and fluorescent lights.
- Lasers: The noble gases are used in lasers, such as excimer lasers and helium-neon lasers.
- Medicine: The noble gases are used in medicine, such as in magnetic resonance imaging (MRI) machines and radiation therapy.
Noble Gases in Lighting
The noble gases are used in lighting, such as in neon signs and fluorescent lights. Neon signs, for example, use electrified glass tubes filled with neon gas to produce a bright, glowing light. Fluorescent lights, on the other hand, use a combination of argon and mercury vapor to produce a bright, white light.
Noble Gases in Lasers
The noble gases are used in lasers, such as excimer lasers and helium-neon lasers. Excimer lasers, for example, use a combination of argon, krypton, and xenon gases to produce a high-powered, ultraviolet laser beam. Helium-neon lasers, on the other hand, use a combination of helium and neon gases to produce a low-powered, red laser beam.
Challenges and Future Directions

Despite the many applications of the noble gases, there are still many challenges and future directions in the field. One of the challenges is the development of new and more efficient ways to produce and use the noble gases. Another challenge is the study of the properties and behavior of the noble gases at the atomic and molecular level.
Some of the future directions in the field include:
- Development of new noble gas-based technologies, such as noble gas-based lasers and lighting systems.
- Study of the properties and behavior of the noble gases at the atomic and molecular level, such as their electron configuration and chemical reactivity.
- Exploration of the potential applications of the noble gases in fields such as medicine and materials science.
Conclusion
In conclusion, the noble gases are a unique group of elements that are known for their unreactive nature. Their stability can be attributed to their electron configuration and electron shells, which make it difficult for them to form bonds with other elements. Despite their unreactive nature, the noble gases have many important applications in various fields, including lighting, lasers, and medicine. However, there are still many challenges and future directions in the field, such as the development of new and more efficient ways to produce and use the noble gases, and the study of their properties and behavior at the atomic and molecular level.
What are the noble gases?
+The noble gases are a group of elements in the periodic table that are known for their unreactive nature. They include elements such as helium, neon, argon, krypton, xenon, and radon.
Why are the noble gases stable?
+The noble gases are stable because of their electron configuration and electron shells. They have a full outer energy level, which makes it difficult for them to form bonds with other elements.
What are some of the applications of the noble gases?
+The noble gases have many important applications in various fields, including lighting, lasers, and medicine. They are used in neon signs, fluorescent lights, excimer lasers, and helium-neon lasers, among other things.