Noble gases are a group of elements that have been a subject of interest for scientists and researchers for centuries. Located in the far right column of the periodic table, these elements are known for their unique properties and behaviors. One of the most distinctive characteristics of noble gases is their tendency to not form bonds with other elements. But why is this the case?
What are Noble Gases?

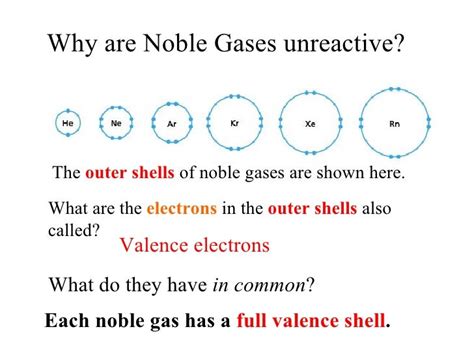
Noble gases are a group of six elements: Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe), and Radon (Rn). They are located in the far right column of the periodic table and are also known as the inert gases. This name is derived from their inability to form bonds with other elements, which makes them "inert" in many chemical reactions.
Electron Configuration and Bonding

The electron configuration of an element plays a crucial role in determining its bonding behavior. In the case of noble gases, their electron configuration is unique in that they have a full outer energy level. This means that their outermost energy level is completely filled with electrons, which makes it difficult for them to form bonds with other elements.
When an element forms a bond with another element, it does so by sharing or exchanging electrons. However, in the case of noble gases, their full outer energy level means that they have no electrons to share or exchange. As a result, they tend to not form bonds with other elements.
Exceptions to the Rule
While noble gases tend to not form bonds with other elements, there are some exceptions to this rule. For example, Xenon and Radon have been known to form bonds with other elements under certain conditions. This is because these elements have a slightly different electron configuration than the other noble gases, which makes it possible for them to form bonds.
In addition, noble gases can also form bonds with other elements when they are subjected to high pressures and temperatures. This is because these conditions can cause the electrons in the noble gas to become excited and form bonds with other elements.
Uses of Noble Gases

Despite their tendency to not form bonds with other elements, noble gases have a number of important uses. For example, Neon is used in neon signs, while Argon is used in light bulbs. Krypton and Xenon are used in high-intensity lamps, such as those used in photography and movie projectors.
In addition, noble gases are also used in a number of scientific applications, such as in the study of superconductivity and superfluidity. They are also used in the production of semiconductors and other electronic components.
Medical Applications
Noble gases also have a number of medical applications. For example, Xenon is used as an anesthetic gas, while Radon is used in the treatment of certain types of cancer. In addition, noble gases are also used in the production of medical imaging agents, such as those used in MRI and PET scans.
Environmental Impact

Noble gases can have a significant environmental impact, particularly when they are released into the atmosphere. For example, Xenon and Radon are both radioactive, which means that they can contaminate the air and water if they are released in large quantities.
In addition, noble gases can also contribute to climate change. For example, Xenon and Krypton are both potent greenhouse gases, which means that they can trap heat in the atmosphere and contribute to global warming.
Conservation Efforts
As a result of their environmental impact, there are a number of conservation efforts underway to reduce the release of noble gases into the atmosphere. For example, the production of semiconductors and other electronic components is being modified to reduce the use of noble gases. In addition, there are also efforts underway to develop new technologies that can capture and recycle noble gases, rather than releasing them into the atmosphere.
Conclusion and Future Directions

In conclusion, noble gases are a group of elements that tend to not form bonds with other elements due to their unique electron configuration. Despite this, they have a number of important uses and applications, including in the production of semiconductors and other electronic components, as well as in medical and scientific applications.
As we move forward, it will be important to continue to develop new technologies and conservation efforts to reduce the release of noble gases into the atmosphere and mitigate their environmental impact. This will require continued research and development in the fields of chemistry and physics, as well as a commitment to sustainability and environmental stewardship.
We invite you to share your thoughts and opinions on the topic of noble gases and their uses. How do you think we can reduce the environmental impact of these elements? Share your comments and suggestions below.
What are noble gases?
+Noble gases are a group of six elements: Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe), and Radon (Rn). They are located in the far right column of the periodic table and are also known as the inert gases.
Why do noble gases tend to not form bonds with other elements?
+Noble gases tend to not form bonds with other elements because they have a full outer energy level, which means that they have no electrons to share or exchange.
What are some of the uses of noble gases?
+Noble gases have a number of important uses, including in the production of semiconductors and other electronic components, as well as in medical and scientific applications.