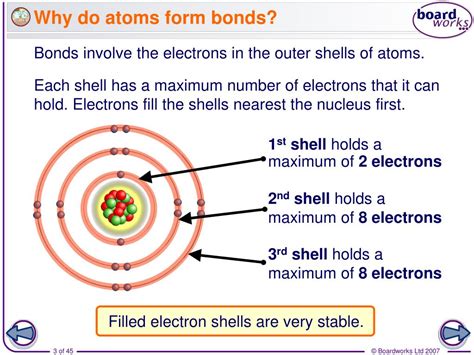
Atoms, the building blocks of matter, are the fundamental units of chemical elements. They are the smallest units of a chemical element that still retain the properties of that element. Atoms are made up of protons, neutrons, and electrons, and they are always in motion. One of the most fascinating aspects of atoms is their ability to form chemical bonds with other atoms. But why do atoms form chemical bonds with other atoms?
Atoms form chemical bonds with other atoms to achieve a more stable electronic configuration. In other words, atoms form bonds to gain or lose electrons to complete their outer energy level. This is because atoms are most stable when their outer energy level is fully occupied with electrons. When an atom's outer energy level is not fully occupied, it becomes reactive and tends to gain or lose electrons to achieve a more stable configuration.
What is a Chemical Bond?

A chemical bond is a lasting attraction between atoms, ions, or molecules that enables the formation of chemical compounds. It is a result of the interaction between the electrons of two or more atoms. Chemical bonds can be classified into several types, including covalent bonds, ionic bonds, and metallic bonds.
Covalent bonds are formed when two or more atoms share one or more pairs of electrons to achieve a more stable electronic configuration. This type of bond is commonly found in molecules, where two or more atoms are chemically bonded together. Ionic bonds, on the other hand, are formed when one or more electrons are transferred from one atom to another, resulting in the formation of ions with opposite charges. These ions are then attracted to each other, forming a chemical bond. Metallic bonds are formed when a large number of atoms share electrons in a "sea" of electrons, resulting in a strong and delocalized bond.
Types of Chemical Bonds
- Covalent bonds: formed by sharing electrons between atoms
- Ionic bonds: formed by transferring electrons between atoms
- Metallic bonds: formed by sharing electrons in a "sea" of electrons
Why Atoms Form Covalent Bonds

Atoms form covalent bonds to achieve a more stable electronic configuration. When two or more atoms share one or more pairs of electrons, they form a covalent bond. This type of bond is commonly found in molecules, where two or more atoms are chemically bonded together. Covalent bonds are typically formed between nonmetal atoms, such as carbon, nitrogen, and oxygen.
The formation of covalent bonds involves the sharing of electrons between atoms. When two atoms share electrons, they form a covalent bond. The shared electrons are attracted to the nuclei of both atoms, resulting in a strong and stable bond. Covalent bonds can be polar or nonpolar, depending on the difference in electronegativity between the atoms involved.
Characteristics of Covalent Bonds
- Formed by sharing electrons between atoms
- Typically found in molecules
- Can be polar or nonpolar
- Involves the sharing of one or more pairs of electrons
Why Atoms Form Ionic Bonds

Atoms form ionic bonds to achieve a more stable electronic configuration. When one or more electrons are transferred from one atom to another, the resulting ions are attracted to each other, forming a chemical bond. Ionic bonds are typically formed between metal and nonmetal atoms.
The formation of ionic bonds involves the transfer of electrons between atoms. When a metal atom loses one or more electrons, it becomes a positively charged ion, known as a cation. When a nonmetal atom gains one or more electrons, it becomes a negatively charged ion, known as an anion. The electrostatic attraction between the cation and anion results in the formation of an ionic bond.
Characteristics of Ionic Bonds
- Formed by transferring electrons between atoms
- Typically found in ionic compounds
- Involves the transfer of one or more electrons
- Results in the formation of ions with opposite charges
Why Atoms Form Metallic Bonds

Atoms form metallic bonds to achieve a more stable electronic configuration. When a large number of atoms share electrons in a "sea" of electrons, they form a metallic bond. This type of bond is commonly found in metals, where a large number of atoms are chemically bonded together.
The formation of metallic bonds involves the sharing of electrons in a "sea" of electrons. When a large number of atoms are brought together, their electrons become delocalized, resulting in a strong and delocalized bond. Metallic bonds are typically found in metals, where the atoms are arranged in a regular pattern, known as a crystal lattice.
Characteristics of Metallic Bonds
- Formed by sharing electrons in a "sea" of electrons
- Typically found in metals
- Involves the delocalization of electrons
- Results in a strong and delocalized bond
In conclusion, atoms form chemical bonds with other atoms to achieve a more stable electronic configuration. The type of bond formed depends on the atoms involved and the number of electrons shared or transferred. Understanding the formation of chemical bonds is essential for understanding the properties and behavior of molecules and ionic compounds.
What is a chemical bond?
+A chemical bond is a lasting attraction between atoms, ions, or molecules that enables the formation of chemical compounds.
Why do atoms form covalent bonds?
+Atoms form covalent bonds to achieve a more stable electronic configuration by sharing electrons between atoms.
What is the difference between ionic and covalent bonds?
+Ionic bonds are formed by transferring electrons between atoms, while covalent bonds are formed by sharing electrons between atoms.
We hope this article has helped you understand the importance of chemical bonds and why atoms form them. If you have any questions or comments, please feel free to share them with us.