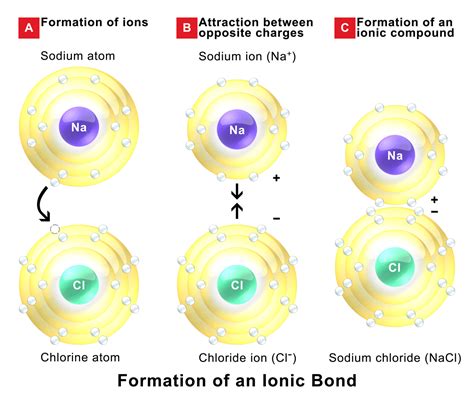
The world of chemistry is fascinating, and one of the most interesting aspects is the formation of ionic compounds. Ionic compounds are formed when two or more elements share electrons to create a chemical bond. But, have you ever wondered which elements can bond together to form ionic compounds? In this article, we will explore the world of ionic compound formation and discuss which elements can bond together.
Ionic compounds are typically formed between metals and nonmetals. Metals are elements that are found on the left side of the periodic table, while nonmetals are elements that are found on the right side. When a metal and a nonmetal bond together, they form an ionic compound. But, not all metals and nonmetals can bond together. There are certain rules that govern which elements can form ionic compounds.
Understanding the Periodic Table

Before we dive into which elements can bond together, it's essential to understand the periodic table. The periodic table is a chart that shows the elements in order of their atomic number. The elements are arranged in rows called periods and columns called groups. The elements in each group have similar properties, and the elements in each period have similar electron configurations.
Metals, Nonmetals, and Metalloids
The periodic table is divided into three main categories: metals, nonmetals, and metalloids. Metals are elements that are found on the left side of the periodic table, while nonmetals are elements that are found on the right side. Metalloids are elements that are found on the border between metals and nonmetals. They have properties of both metals and nonmetals.
Which Elements Can Bond Together?

Now that we understand the periodic table, let's discuss which elements can bond together. Ionic compounds are typically formed between metals and nonmetals. Metals have a tendency to lose electrons, while nonmetals have a tendency to gain electrons. When a metal and a nonmetal bond together, the metal loses electrons to form a cation, while the nonmetal gains electrons to form an anion.
Examples of Ionic Compounds
Here are some examples of ionic compounds:
- Sodium chloride (NaCl) - formed from sodium (a metal) and chlorine (a nonmetal)
- Calcium carbonate (CaCO3) - formed from calcium (a metal) and carbon (a nonmetal) and oxygen (a nonmetal)
- Aluminum oxide (Al2O3) - formed from aluminum (a metal) and oxygen (a nonmetal)
Rules for Ionic Compound Formation

There are certain rules that govern which elements can form ionic compounds. Here are some of the rules:
- Metals tend to lose electrons to form cations.
- Nonmetals tend to gain electrons to form anions.
- The number of electrons lost or gained by an element is determined by its electron configuration.
- The charges on the ions must balance each other out.
- The formula of an ionic compound is determined by the charges on the ions.
Exceptions to the Rules
While these rules govern the formation of ionic compounds, there are some exceptions. For example:
- Hydrogen can form ionic compounds with nonmetals, even though it is a nonmetal itself.
- Some metals can form covalent bonds with nonmetals, instead of ionic bonds.
Properties of Ionic Compounds

Ionic compounds have some unique properties, including:
- High melting and boiling points
- Hardness and brittleness
- Conductivity
- Solubility in water
Examples of Ionic Compounds with Unique Properties
Here are some examples of ionic compounds with unique properties:
- Sodium chloride (NaCl) is a good conductor of electricity.
- Calcium carbonate (CaCO3) is hard and brittle.
- Aluminum oxide (Al2O3) is highly soluble in water.
Conclusion
In conclusion, ionic compounds are formed when metals and nonmetals bond together. The periodic table is essential in understanding which elements can form ionic compounds. Metals tend to lose electrons to form cations, while nonmetals tend to gain electrons to form anions. There are certain rules that govern the formation of ionic compounds, but there are also some exceptions. Ionic compounds have unique properties, including high melting and boiling points, hardness and brittleness, conductivity, and solubility in water.
What is an ionic compound?
+An ionic compound is a type of chemical compound that is formed when a metal and a nonmetal bond together.
What are the rules for ionic compound formation?
+The rules for ionic compound formation include: metals tend to lose electrons to form cations, nonmetals tend to gain electrons to form anions, the number of electrons lost or gained by an element is determined by its electron configuration, the charges on the ions must balance each other out, and the formula of an ionic compound is determined by the charges on the ions.
What are some examples of ionic compounds?
+Some examples of ionic compounds include sodium chloride (NaCl), calcium carbonate (CaCO3), and aluminum oxide (Al2O3).