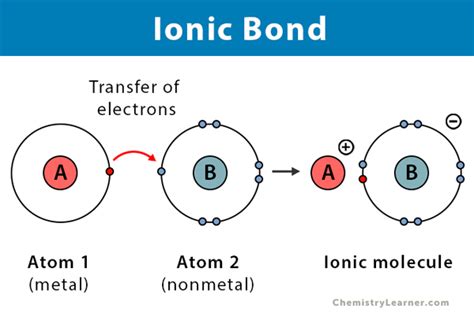
Ionic bonds are a type of chemical bond that forms between two atoms that have a large difference in electronegativity. This type of bond is typically formed between a metal atom and a nonmetal atom. In this article, we will explore which atoms form ionic bonds and the factors that influence the formation of these bonds.
What are Ionic Bonds?

Ionic bonds are a type of chemical bond that forms between two atoms that have a large difference in electronegativity. Electronegativity is the measure of an atom's ability to attract electrons in a covalent bond. When two atoms with a large difference in electronegativity come together, the atom with the higher electronegativity pulls the shared electrons towards itself, resulting in the formation of ions. The atom that loses electrons becomes a positively charged ion, known as a cation, while the atom that gains electrons becomes a negatively charged ion, known as an anion. The electrostatic attraction between the cation and anion holds them together, forming an ionic bond.
Atoms that Form Ionic Bonds
Ionic bonds typically form between metal atoms and nonmetal atoms. Metal atoms tend to lose electrons to form cations, while nonmetal atoms tend to gain electrons to form anions. Some examples of atoms that form ionic bonds include:
- Alkali metals (Group 1): Lithium (Li), Sodium (Na), Potassium (K), Rubidium (Rb), Caesium (Cs)
- Alkaline earth metals (Group 2): Magnesium (Mg), Calcium (Ca), Strontium (Sr), Barium (Ba)
- Halogens (Group 17): Fluorine (F), Chlorine (Cl), Bromine (Br), Iodine (I)
- Noble gases (Group 18): Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe)
These atoms tend to form ionic bonds with each other, resulting in the formation of ionic compounds such as sodium chloride (NaCl), calcium carbonate (CaCO3), and magnesium oxide (MgO).
Factors that Influence the Formation of Ionic Bonds

The formation of ionic bonds is influenced by several factors, including:
- Electronegativity difference: The larger the difference in electronegativity between the two atoms, the more likely they are to form an ionic bond.
- Ionization energy: The ease with which an atom loses electrons to form a cation affects its ability to form ionic bonds.
- Electron affinity: The ease with which an atom gains electrons to form an anion affects its ability to form ionic bonds.
- Atomic size: The size of the atoms involved in the bond affects the strength of the ionic bond.
These factors influence the strength and stability of the ionic bond, with stronger bonds forming between atoms with a large electronegativity difference and weaker bonds forming between atoms with a smaller electronegativity difference.
Examples of Ionic Bonds
Ionic bonds are found in many everyday substances, including:
- Table salt (sodium chloride, NaCl)
- Baking soda (sodium bicarbonate, NaHCO3)
- Calcium carbonate (CaCO3)
- Magnesium oxide (MgO)
- Potassium nitrate (KNO3)
These substances are all composed of ions that are held together by ionic bonds, resulting in the formation of a crystalline lattice structure.
Properties of Ionic Bonds

Ionic bonds have several properties that distinguish them from other types of chemical bonds, including:
- High melting and boiling points: Ionic bonds are typically strong and rigid, resulting in high melting and boiling points.
- Hardness and brittleness: Ionic compounds are often hard and brittle, due to the rigid structure of the ionic lattice.
- Solubility: Ionic compounds tend to be soluble in water, due to the ability of the ions to interact with water molecules.
- Conductivity: Ionic compounds can conduct electricity when dissolved in water, due to the movement of ions.
These properties make ionic bonds an important type of chemical bond in many areas of chemistry and physics.
Importance of Ionic Bonds
Ionic bonds play a crucial role in many areas of science and technology, including:
- Biology: Ionic bonds are important in many biological molecules, such as DNA and proteins.
- Materials science: Ionic bonds are used in the development of many materials, such as ceramics and glass.
- Energy: Ionic bonds are used in the development of energy storage devices, such as batteries.
- Environmental science: Ionic bonds are important in many environmental processes, such as the formation of minerals and the behavior of pollutants.
In conclusion, ionic bonds are an important type of chemical bond that forms between atoms with a large difference in electronegativity. Understanding which atoms form ionic bonds and the factors that influence the formation of these bonds is crucial in many areas of science and technology.
We hope you found this article informative and helpful in understanding the concept of ionic bonds. If you have any questions or comments, please feel free to share them with us!
What is the main difference between ionic bonds and covalent bonds?
+The main difference between ionic bonds and covalent bonds is the way in which the atoms share electrons. In ionic bonds, the atoms transfer electrons to form ions, while in covalent bonds, the atoms share electrons to form a molecule.
Which atoms tend to form ionic bonds?
+Metal atoms tend to lose electrons to form cations, while nonmetal atoms tend to gain electrons to form anions. Some examples of atoms that form ionic bonds include alkali metals, alkaline earth metals, halogens, and noble gases.
What are some examples of ionic bonds in everyday substances?
+Examples of ionic bonds in everyday substances include table salt (sodium chloride), baking soda (sodium bicarbonate), calcium carbonate, magnesium oxide, and potassium nitrate.