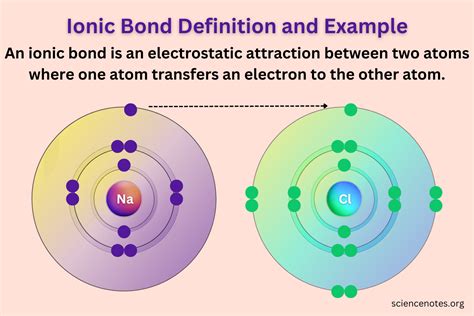
Ionic bonds are a type of chemical bond that involves the transfer of electrons between atoms, resulting in the formation of ions with opposite charges. These ions are attracted to each other, forming a strong electrostatic bond. However, not all ionic bonds are created equal, and some ionic bond pairs form stronger bonds than others.

In this article, we will explore the factors that determine the strength of an ionic bond and identify some of the strongest ionic bond pairs.
Factors Affecting Ionic Bond Strength
Several factors contribute to the strength of an ionic bond, including:
- Electronegativity difference: The greater the difference in electronegativity between the two atoms, the stronger the ionic bond.
- Ion size: Smaller ions tend to form stronger bonds than larger ions.
- Charge: Ions with higher charges tend to form stronger bonds than those with lower charges.
- Polarizability: Ions with high polarizability tend to form stronger bonds than those with low polarizability.
Electronegativity Difference
Electronegativity is a measure of an atom's ability to attract electrons. When two atoms with different electronegativities form an ionic bond, the atom with the higher electronegativity tends to pull the shared electrons towards itself, resulting in a stronger bond. For example, the electronegativity difference between sodium (Na) and chlorine (Cl) is 2.1, which is relatively large, resulting in a strong ionic bond.
Strongest Ionic Bond Pairs
Based on the factors mentioned above, some of the strongest ionic bond pairs include:
- Sodium (Na) and Chlorine (Cl): The electronegativity difference between sodium and chlorine is 2.1, resulting in a strong ionic bond.
- Calcium (Ca) and Oxygen (O): The electronegativity difference between calcium and oxygen is 2.5, resulting in a strong ionic bond.
- Magnesium (Mg) and Fluorine (F): The electronegativity difference between magnesium and fluorine is 3.2, resulting in a very strong ionic bond.
- Aluminum (Al) and Oxygen (O): The electronegativity difference between aluminum and oxygen is 2.5, resulting in a strong ionic bond.

These ionic bond pairs are commonly found in nature and are responsible for the formation of many minerals and compounds.
Examples of Strong Ionic Bonds in Nature
- Table salt: Sodium chloride (NaCl) is a classic example of a strong ionic bond. The electronegativity difference between sodium and chlorine is 2.1, resulting in a strong bond that is resistant to heat and pressure.
- Calcite: Calcium carbonate (CaCO3) is a mineral that is composed of calcium, carbon, and oxygen atoms. The electronegativity difference between calcium and oxygen is 2.5, resulting in a strong ionic bond.
- Corundum: Aluminum oxide (Al2O3) is a mineral that is composed of aluminum and oxygen atoms. The electronegativity difference between aluminum and oxygen is 2.5, resulting in a strong ionic bond.
Importance of Ionic Bonds
Ionic bonds play a crucial role in the formation of many minerals and compounds. They are also responsible for the unique properties of many materials, such as their hardness, melting point, and solubility.

In conclusion, ionic bonds are a type of chemical bond that involves the transfer of electrons between atoms, resulting in the formation of ions with opposite charges. The strength of an ionic bond depends on several factors, including electronegativity difference, ion size, charge, and polarizability. Some of the strongest ionic bond pairs include sodium and chlorine, calcium and oxygen, magnesium and fluorine, and aluminum and oxygen. These ionic bond pairs are commonly found in nature and are responsible for the formation of many minerals and compounds.
We hope this article has provided you with a better understanding of ionic bonds and their importance in nature. If you have any questions or comments, please feel free to share them with us.
What is an ionic bond?
+An ionic bond is a type of chemical bond that involves the transfer of electrons between atoms, resulting in the formation of ions with opposite charges.
What factors affect the strength of an ionic bond?
+The strength of an ionic bond depends on several factors, including electronegativity difference, ion size, charge, and polarizability.
What are some examples of strong ionic bonds in nature?
+Some examples of strong ionic bonds in nature include table salt (sodium chloride), calcite (calcium carbonate), and corundum (aluminum oxide).