Transition metals are a group of metals that are found in the d-block of the periodic table. They are known for their ability to form ions with different charges, which is a result of the partial filling of their d subshells. This property makes them extremely versatile and useful in a wide range of applications, from catalysis to electronics.
One of the key characteristics of transition metals is their ability to form ions with different charges. This is because the electrons in their d subshells are not completely filled, which allows them to lose or gain electrons to form ions with different charges. For example, iron can form ions with charges of +2 and +3, while copper can form ions with charges of +1 and +2. This ability to form ions with different charges makes transition metals extremely useful in a wide range of applications.
How Transition Metals Form Ions

Transition metals form ions through a process called ionization. Ionization occurs when an atom loses or gains electrons to form an ion. In the case of transition metals, the electrons in their d subshells are not completely filled, which makes it easy for them to lose or gain electrons to form ions.
For example, when an iron atom loses two electrons, it forms an iron(II) ion with a charge of +2. Similarly, when a copper atom loses one electron, it forms a copper(I) ion with a charge of +1. The ability of transition metals to form ions with different charges makes them extremely useful in a wide range of applications.
The Factors That Influence Ion Formation
There are several factors that influence the formation of ions by transition metals. One of the key factors is the size of the ion. Smaller ions tend to have a higher charge density, which makes them more stable. This is why ions with higher charges tend to be more stable than ions with lower charges.
Another factor that influences ion formation is the electron configuration of the metal. Metals with a partially filled d subshell tend to form ions with different charges more easily than metals with a fully filled d subshell. This is why transition metals tend to form ions with different charges more easily than other metals.
The Properties of Transition Metal Ions

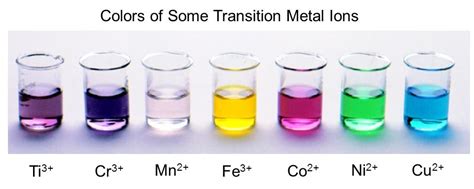
Transition metal ions have several unique properties that make them extremely useful in a wide range of applications. One of the key properties is their ability to form colored ions. Transition metal ions tend to form colored ions due to the presence of unpaired electrons in their d subshells. This is why many transition metal compounds are brightly colored.
Another property of transition metal ions is their ability to form complexes. Transition metal ions tend to form complexes with other ions or molecules, which can have a wide range of properties. For example, some transition metal complexes are extremely stable, while others are highly reactive.
The Applications of Transition Metal Ions
Transition metal ions have a wide range of applications in fields such as catalysis, electronics, and medicine. One of the key applications is in catalysis, where transition metal ions are used to speed up chemical reactions. For example, palladium ions are used to catalyze the hydrogenation of alkenes.
Another application of transition metal ions is in electronics, where they are used to make a wide range of devices, including transistors and solar cells. For example, copper ions are used to make the wiring in electronic devices.
The Biological Importance of Transition Metal Ions

Transition metal ions play a crucial role in many biological processes. One of the key roles is in the transport of oxygen in the blood. Iron ions are used to make hemoglobin, which is the protein that carries oxygen in the blood.
Another role of transition metal ions is in the functioning of enzymes. Many enzymes require transition metal ions to function properly. For example, zinc ions are required for the functioning of many enzymes, including carbonic anhydrase.
The Toxicity of Transition Metal Ions
While transition metal ions are essential for many biological processes, they can also be toxic in high concentrations. For example, high levels of iron ions can cause damage to the liver and other organs.
Another example is copper ions, which can cause damage to the kidneys and other organs if they are present in high concentrations.
Conclusion

In conclusion, transition metal ions are extremely versatile and useful in a wide range of applications. Their ability to form ions with different charges makes them useful in fields such as catalysis, electronics, and medicine.
However, transition metal ions can also be toxic in high concentrations, which makes it essential to handle them with care. Overall, the study of transition metal ions is an important area of research that continues to have a significant impact on many fields.
We hope this article has provided you with a comprehensive understanding of transition metal ions and their importance in various fields. If you have any questions or comments, please feel free to share them with us.
What are transition metal ions?
+Transition metal ions are ions that are formed by transition metals, which are a group of metals that are found in the d-block of the periodic table.
What is the main characteristic of transition metal ions?
+The main characteristic of transition metal ions is their ability to form ions with different charges.
What are some of the applications of transition metal ions?
+Transition metal ions have a wide range of applications in fields such as catalysis, electronics, and medicine.