Elements that form anions are typically found on the right-hand side of the periodic table, with the exception of hydrogen. Anions are negatively charged ions that are formed when an atom gains one or more electrons. In chemistry, the elements that commonly form anions are the nonmetals, which are located in the p-block of the periodic table.

Elements in Group 17 (VIIA) of the periodic table, also known as the halogens, readily form anions. These elements include fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). When a halogen atom gains an electron, it forms a negatively charged ion with a -1 charge. For example, a chlorine atom (Cl) gains an electron to form a chloride ion (Cl-).
Elements in Group 16 (VIA) of the periodic table, also known as the chalcogens, can also form anions. These elements include oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and polonium (Po). When a chalcogen atom gains two electrons, it forms a negatively charged ion with a -2 charge. For example, an oxygen atom (O) gains two electrons to form an oxide ion (O2-).
Elements in Group 15 (VA) of the periodic table, also known as the pnictogens, can form anions with a -3 charge. These elements include nitrogen (N), phosphorus (P), arsenic (As), antimony (Sb), and bismuth (Bi). When a pnictogen atom gains three electrons, it forms a negatively charged ion. For example, a nitrogen atom (N) gains three electrons to form a nitride ion (N3-).
In addition to these groups, other nonmetal elements can also form anions. For example, carbon (C) can form an anion with a -4 charge, known as a carbide ion (C4-). Similarly, boron (B) can form an anion with a -3 charge, known as a boride ion (B3-).
Why Do Elements Form Anions?
Elements form anions to achieve a stable electronic configuration. When an atom gains one or more electrons, it fills its outermost energy level, resulting in a stable arrangement of electrons. This is known as the octet rule, which states that atoms tend to gain, lose, or share electrons to achieve a full outer energy level with eight electrons.

The formation of anions is also influenced by the electronegativity of an element. Electronegativity is a measure of an atom's ability to attract electrons. Elements with high electronegativity values, such as fluorine and oxygen, tend to form anions more readily than elements with low electronegativity values.
Common Anions in Chemistry
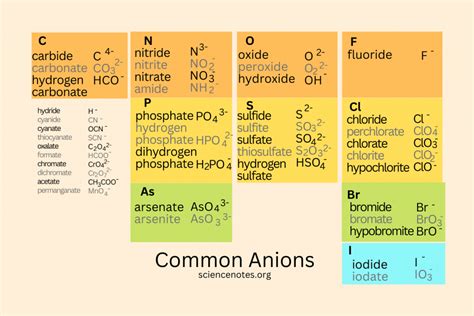
There are many common anions in chemistry, including:
- Halide ions: F-, Cl-, Br-, I-, and At-
- Oxide ions: O2- and OH-
- Sulfide ions: S2- and HS-
- Nitride ions: N3- and NH2-
- Phosphate ions: PO43- and HPO42-
- Carbonate ions: CO32- and HCO3-
These anions are commonly found in ionic compounds, which are formed when a positively charged ion (cation) combines with a negatively charged ion (anion).
Halide Ions
Halide ions are formed when a halogen atom gains an electron. These ions are commonly found in ionic compounds, such as sodium chloride (NaCl) and potassium bromide (KBr).
- F- (fluoride ion)
- Cl- (chloride ion)
- Br- (bromide ion)
- I- (iodide ion)
- At- (astatide ion)

Oxide Ions
Oxide ions are formed when an oxygen atom gains two electrons. These ions are commonly found in ionic compounds, such as calcium oxide (CaO) and magnesium oxide (MgO).
- O2- (oxide ion)
- OH- (hydroxide ion)

Conclusion
In conclusion, elements that form anions are typically found on the right-hand side of the periodic table, with the exception of hydrogen. These elements, including the halogens, chalcogens, and pnictogens, readily form anions by gaining one or more electrons. The formation of anions is influenced by the electronegativity of an element and the octet rule. Common anions in chemistry include halide ions, oxide ions, sulfide ions, nitride ions, phosphate ions, and carbonate ions.
If you have any questions or need further clarification on the topic, please don't hesitate to ask. Share your thoughts and experiences with anions in the comments section below.
What are anions in chemistry?
+Anions are negatively charged ions that are formed when an atom gains one or more electrons.
Which elements form anions?
+Elements that form anions are typically found on the right-hand side of the periodic table, with the exception of hydrogen. These elements include the halogens, chalcogens, and pnictogens.
What is the octet rule?
+The octet rule states that atoms tend to gain, lose, or share electrons to achieve a full outer energy level with eight electrons.