Understanding the intricacies of chemical bonding is essential for anyone delving into the realm of chemistry. Among the various types of chemical bonds, pi bonds play a crucial role in determining the structure and properties of molecules. In this article, we will explore the concept of pi bonds, their formation through orbitals, and their significance in understanding molecular structure.
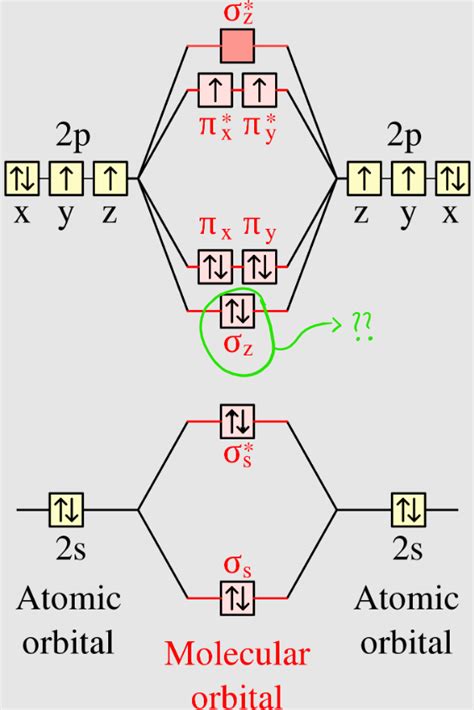
Chemical bonds are primarily classified into two categories: sigma (σ) bonds and pi (π) bonds. While sigma bonds are formed through the head-on overlap of atomic orbitals, pi bonds result from the sideways overlap of parallel p-orbitals. Pi bonds are typically found in molecules with multiple bonds, such as alkenes and alkynes.
What are Pi Bonds?

Pi bonds are a type of covalent bond that involves the sideways overlap of parallel p-orbitals. This overlap results in a nodal plane perpendicular to the bond axis, giving pi bonds their characteristic shape. Pi bonds are typically weaker than sigma bonds due to the less effective overlap of the p-orbitals.
Formation of Pi Bonds
Pi bonds are formed when two parallel p-orbitals overlap sideways. This overlap occurs above and below the bond axis, resulting in a nodal plane perpendicular to the bond axis. The formation of pi bonds is crucial in understanding the structure and properties of molecules with multiple bonds.
For example, in the molecule ethene (C2H4), the carbon atoms form a sigma bond through the head-on overlap of their sp2 hybrid orbitals. Additionally, the carbon atoms form a pi bond through the sideways overlap of their parallel p-orbitals. This pi bond is responsible for the molecule's planar structure and its ability to rotate freely around the bond axis.
Role of Orbitals in Pi Bond Formation

Orbitals play a crucial role in the formation of pi bonds. In molecules with multiple bonds, the atomic orbitals of the atoms involved in bonding hybridize to form molecular orbitals. These molecular orbitals are responsible for the formation of sigma and pi bonds.
In the case of pi bonds, the p-orbitals of the atoms involved in bonding hybridize to form molecular orbitals. The sideways overlap of these molecular orbitals results in the formation of a pi bond. The shape and orientation of the p-orbitals determine the shape and orientation of the pi bond.
Types of Pi Bonds
Pi bonds can be classified into two categories: localized pi bonds and delocalized pi bonds. Localized pi bonds are found in molecules with isolated multiple bonds, such as alkenes and alkynes. Delocalized pi bonds, on the other hand, are found in molecules with conjugated multiple bonds, such as aromatic compounds.
Delocalized pi bonds are formed when the molecular orbitals of adjacent pi bonds overlap, resulting in a delocalized electron cloud. This delocalization of electrons is responsible for the stability and unique properties of aromatic compounds.
Significance of Pi Bonds in Understanding Molecular Structure

Pi bonds play a crucial role in determining the structure and properties of molecules. The presence of pi bonds in a molecule can influence its shape, polarity, and reactivity.
For example, the planar structure of molecules with pi bonds, such as ethene, is due to the nodal plane perpendicular to the bond axis. This planar structure is responsible for the molecule's ability to rotate freely around the bond axis.
In addition, the presence of pi bonds in a molecule can influence its polarity. The sideways overlap of p-orbitals results in a dipole moment perpendicular to the bond axis, contributing to the molecule's overall polarity.
Applications of Pi Bonds
Pi bonds have numerous applications in various fields, including chemistry, materials science, and pharmaceuticals. The unique properties of pi bonds make them essential in understanding the structure and properties of molecules.
For example, the delocalization of electrons in aromatic compounds is responsible for their stability and unique properties. This delocalization is exploited in the design of materials with specific properties, such as conductive polymers.
In conclusion, pi bonds are a crucial aspect of chemical bonding, and their formation through orbitals is essential in understanding molecular structure. The significance of pi bonds in determining the structure and properties of molecules makes them a fundamental concept in chemistry.
Now, we'd love to hear from you! Do you have any questions or topics related to pi bonds you'd like to discuss? Share your thoughts in the comments below, and don't forget to share this article with your friends and colleagues.
What is the main difference between sigma and pi bonds?
+Sigma bonds are formed through the head-on overlap of atomic orbitals, while pi bonds are formed through the sideways overlap of parallel p-orbitals.
What is the role of orbitals in pi bond formation?
+Orbitals play a crucial role in the formation of pi bonds. The p-orbitals of the atoms involved in bonding hybridize to form molecular orbitals, which overlap sideways to form a pi bond.
What is the significance of pi bonds in understanding molecular structure?
+Pi bonds play a crucial role in determining the structure and properties of molecules. The presence of pi bonds can influence a molecule's shape, polarity, and reactivity.