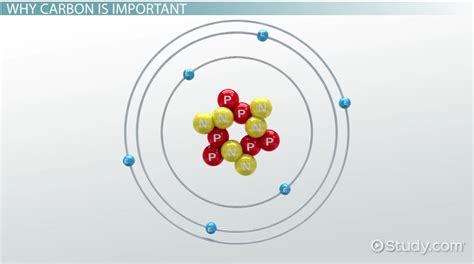
The fascinating world of chemistry! Carbon, a fundamental element of life, has the unique ability to form bonds with numerous other elements. This versatility is the backbone of organic chemistry and the basis of all life on Earth. In this article, we will delve into the four primary types of bonds that carbon forms with other elements.
Carbon, with its six electrons, can form a wide variety of molecules by sharing its electrons with other atoms. This ability to form bonds is known as carbon's tetravalency. The four types of bonds that carbon forms with other elements are: covalent bonds, ionic bonds, hydrogen bonds, and metallic bonds.
Understanding Covalent Bonds

Covalent bonds are the most common type of bond that carbon forms with other elements. In a covalent bond, two or more atoms share one or more pairs of electrons to achieve a stable electronic configuration. This sharing of electrons leads to the formation of a molecule. Covalent bonds can be further classified into two subcategories: polar covalent bonds and nonpolar covalent bonds.
In a polar covalent bond, the electrons are not shared equally between the two atoms. This unequal sharing of electrons leads to the formation of a dipole, where one end of the molecule has a partial positive charge, and the other end has a partial negative charge. Water (H2O) is a classic example of a molecule with polar covalent bonds.
In a nonpolar covalent bond, the electrons are shared equally between the two atoms. This equal sharing of electrons leads to the formation of a molecule with no net dipole moment. Methane (CH4) is an example of a molecule with nonpolar covalent bonds.
Characteristics of Covalent Bonds
- Covalent bonds are typically strong and stable.
- They are formed between nonmetal atoms.
- The atoms involved in a covalent bond share one or more pairs of electrons.
- Covalent bonds can be polar or nonpolar.
Understanding Ionic Bonds

Ionic bonds are formed when one or more electrons are transferred from one atom to another, resulting in the formation of ions with opposite charges. The electrostatic attraction between the positively charged ion (cation) and the negatively charged ion (anion) leads to the formation of an ionic bond. Carbon can form ionic bonds with highly electronegative elements such as oxygen and fluorine.
In an ionic bond, the cation and anion are held together by electrostatic forces. The strength of the ionic bond depends on the magnitude of the charges and the size of the ions involved.
Characteristics of Ionic Bonds
- Ionic bonds are typically strong and rigid.
- They are formed between metal and nonmetal atoms.
- The atoms involved in an ionic bond transfer one or more electrons.
- Ionic bonds are typically formed in compounds with high melting and boiling points.
Understanding Hydrogen Bonds

Hydrogen bonds are a type of intermolecular force that arises between molecules with a hydrogen atom bonded to a highly electronegative atom, such as oxygen, nitrogen, or fluorine. Hydrogen bonds are responsible for the unique properties of water and are essential for the structure and function of biological molecules such as DNA and proteins.
In a hydrogen bond, the hydrogen atom is attracted to the electronegative atom, leading to the formation of a weak bond. Hydrogen bonds are typically weaker than covalent and ionic bonds but play a crucial role in the structure and function of biological molecules.
Characteristics of Hydrogen Bonds
- Hydrogen bonds are typically weak and flexible.
- They are formed between molecules with a hydrogen atom bonded to an electronegative atom.
- Hydrogen bonds are responsible for the unique properties of water and biological molecules.
- Hydrogen bonds are essential for the structure and function of biological molecules.
Understanding Metallic Bonds

Metallic bonds are formed when a large number of atoms share their electrons in a delocalized manner, resulting in the formation of a "sea of electrons." This delocalization of electrons leads to the unique properties of metals, such as their high electrical and thermal conductivity.
In a metallic bond, the electrons are not localized between specific atoms but are free to move throughout the metal lattice. This delocalization of electrons leads to the formation of a strong and flexible bond.
Characteristics of Metallic Bonds
- Metallic bonds are typically strong and flexible.
- They are formed between metal atoms.
- The electrons in a metallic bond are delocalized and free to move throughout the metal lattice.
- Metallic bonds are responsible for the unique properties of metals.
In conclusion, carbon's ability to form bonds with other elements is the basis of all life on Earth. The four primary types of bonds that carbon forms with other elements are covalent bonds, ionic bonds, hydrogen bonds, and metallic bonds. Each type of bond has its unique characteristics and plays a crucial role in the structure and function of molecules.
We hope this article has provided you with a comprehensive understanding of the different types of bonds that carbon forms with other elements. If you have any questions or comments, please feel free to share them below.
What is the most common type of bond that carbon forms with other elements?
+Covalent bonds are the most common type of bond that carbon forms with other elements.
What is the difference between a polar covalent bond and a nonpolar covalent bond?
+In a polar covalent bond, the electrons are not shared equally between the two atoms, leading to the formation of a dipole. In a nonpolar covalent bond, the electrons are shared equally between the two atoms, resulting in no net dipole moment.
What is the role of hydrogen bonds in biological molecules?
+Hydrogen bonds play a crucial role in the structure and function of biological molecules such as DNA and proteins. They are responsible for the unique properties of water and are essential for the formation of secondary and tertiary structures in biological molecules.