Atoms are the basic building blocks of matter, and they are always on the move, interacting with each other in various ways. One of the most fundamental ways atoms interact is through the formation of chemical bonds. Chemical bonds are the attractive and repulsive forces that hold atoms together, and they are responsible for the structure and properties of molecules.
Understanding how atoms form chemical bonds is crucial in chemistry and physics, as it helps us comprehend the behavior of matter at the atomic level. In this article, we will explore the five main ways atoms form chemical bonds, and we will discuss the principles and examples of each type of bond.

1. Covalent Bonds
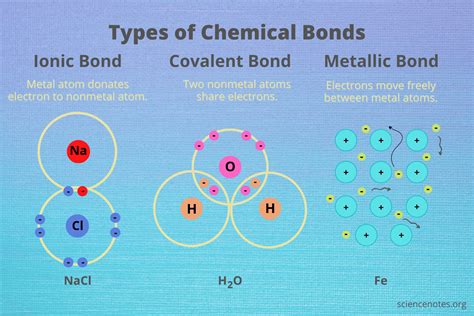
Covalent bonds are one of the most common types of chemical bonds. They form when two or more atoms share one or more pairs of electrons in order to achieve a stable electronic configuration. This sharing of electrons leads to a strong attraction between the atoms, holding them together in a molecule.
Covalent bonds can be polar or nonpolar, depending on the difference in electronegativity between the atoms involved. Polar covalent bonds occur when there is a significant difference in electronegativity, resulting in a partial positive charge on one atom and a partial negative charge on the other. Nonpolar covalent bonds occur when the difference in electronegativity is small, resulting in a more equal sharing of electrons.
Examples of covalent bonds include the bonds between carbon and hydrogen atoms in methane (CH4), and the bonds between oxygen and hydrogen atoms in water (H2O).
Types of Covalent Bonds
There are several types of covalent bonds, including:
- Sigma (σ) bonds: These bonds form when the overlap of atomic orbitals is symmetrical around the bond axis.
- Pi (π) bonds: These bonds form when the overlap of atomic orbitals is asymmetrical around the bond axis.
- Delta (δ) bonds: These bonds form when the overlap of atomic orbitals is symmetrical around the bond axis, but with a different orientation than sigma bonds.
2. Ionic Bonds
Ionic bonds form when one or more electrons are transferred from one atom to another, resulting in the formation of ions with opposite charges. The electrostatic attraction between the positively charged ion (cation) and the negatively charged ion (anion) holds them together in a molecule.
Ionic bonds typically occur between metals and nonmetals, and they are commonly found in salts and other inorganic compounds. Examples of ionic bonds include the bonds between sodium and chlorine atoms in table salt (NaCl), and the bonds between calcium and oxygen atoms in limestone (CaCO3).

3. Hydrogen Bonds
Hydrogen bonds are a type of intermolecular force that arises from the interaction between a hydrogen atom bonded to a highly electronegative atom (such as oxygen, nitrogen, or fluorine) and another electronegative atom. This interaction is weak compared to covalent and ionic bonds, but it plays a crucial role in the structure and properties of many biological molecules, such as DNA and proteins.
Hydrogen bonds are responsible for the high boiling point of water and the low vapor pressure of hydrogen fluoride. They also play a key role in the formation of ice crystals and the structure of biological membranes.
4. Van der Waals Bonds
Van der Waals bonds are a type of intermolecular force that arises from the interaction between temporary dipoles in molecules. These temporary dipoles form when the electrons in a molecule are not evenly distributed, creating a partial positive charge on one side of the molecule and a partial negative charge on the other.
Van der Waals bonds are responsible for the physical properties of many substances, such as melting point, boiling point, and viscosity. They are also responsible for the adhesion of molecules to surfaces and the cohesion of molecules in the liquid phase.

5. Metallic Bonds
Metallic bonds form in metals and metal alloys, and they arise from the interaction between the electrons in the outermost energy level of the metal atoms. In metals, the electrons are delocalized, meaning they are free to move throughout the solid. This delocalization of electrons leads to a "sea of electrons" that surrounds the positively charged metal ions, holding them together in a solid.
Metallic bonds are responsible for the high electrical and thermal conductivity of metals, as well as their malleability and ductility.
In conclusion, the five main ways atoms form chemical bonds are covalent bonds, ionic bonds, hydrogen bonds, van der Waals bonds, and metallic bonds. Each type of bond has its unique characteristics and plays a crucial role in the structure and properties of molecules.
What is the difference between a covalent bond and an ionic bond?
+A covalent bond forms when two or more atoms share one or more pairs of electrons, while an ionic bond forms when one or more electrons are transferred from one atom to another, resulting in the formation of ions with opposite charges.
What is the role of hydrogen bonds in biological molecules?
+Hydrogen bonds play a crucial role in the structure and properties of biological molecules, such as DNA and proteins. They are responsible for the high melting point of DNA and the low vapor pressure of hydrogen fluoride.
What is the difference between a van der Waals bond and a metallic bond?
+A van der Waals bond is a type of intermolecular force that arises from the interaction between temporary dipoles in molecules, while a metallic bond forms in metals and metal alloys, and arises from the interaction between the electrons in the outermost energy level of the metal atoms.