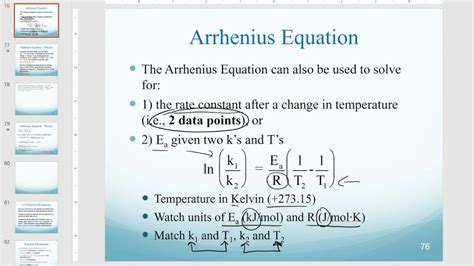
The Arrhenius equation is a fundamental concept in chemistry that describes the temperature dependence of reaction rates. Understanding this equation is crucial for chemists and researchers to predict and analyze the behavior of chemical reactions. In this article, we will delve into the two key points of the Arrhenius equation, exploring its significance and relevance in the field of chemistry.
The Arrhenius equation is a mathematical expression that relates the rate constant of a chemical reaction to the temperature at which the reaction occurs. This equation is named after the Swedish chemist Svante Arrhenius, who first proposed it in the late 19th century. The equation is widely used to analyze and predict the kinetics of chemical reactions, and it has become a cornerstone of chemical kinetics.
Key Point 1: The Activation Energy

The first key point of the Arrhenius equation is the concept of activation energy. Activation energy is the minimum amount of energy required for a chemical reaction to occur. It is a critical parameter in the Arrhenius equation, as it determines the rate constant of the reaction. The activation energy is typically represented by the symbol Ea, and it is measured in units of energy, such as joules or calories.
The activation energy is a barrier that must be overcome for a reaction to proceed. When the reactants have sufficient energy to overcome this barrier, they can transform into products. The activation energy is a measure of the energy required to initiate the reaction, and it is a fundamental property of the reaction itself.
Factors Affecting Activation Energy
The activation energy of a reaction is influenced by several factors, including:
- The nature of the reactants: Different reactants have different activation energies, depending on their molecular structure and properties.
- The reaction mechanism: The activation energy can vary depending on the specific mechanism of the reaction.
- The catalyst: The presence of a catalyst can lower the activation energy, making the reaction faster and more efficient.
- The temperature: The activation energy can be affected by changes in temperature.
Key Point 2: The Pre-Exponential Factor

The second key point of the Arrhenius equation is the pre-exponential factor, also known as the frequency factor. The pre-exponential factor is a constant that is related to the frequency of collisions between reactant molecules. It is typically represented by the symbol A, and it is measured in units of concentration, such as moles per liter.
The pre-exponential factor is a measure of the number of collisions that occur between reactant molecules per unit time. It is a critical parameter in the Arrhenius equation, as it determines the rate constant of the reaction. The pre-exponential factor is influenced by several factors, including:
- The concentration of reactants: The pre-exponential factor increases with increasing reactant concentration.
- The reaction mechanism: The pre-exponential factor can vary depending on the specific mechanism of the reaction.
- The temperature: The pre-exponential factor can be affected by changes in temperature.
Relationship Between Activation Energy and Pre-Exponential Factor
The activation energy and pre-exponential factor are related through the Arrhenius equation. The equation shows that the rate constant of a reaction is proportional to the pre-exponential factor and exponentially dependent on the activation energy. This means that the rate constant increases with increasing pre-exponential factor and decreases with increasing activation energy.
The Arrhenius equation is a powerful tool for analyzing and predicting the kinetics of chemical reactions. By understanding the two key points of the equation, researchers can gain valuable insights into the behavior of chemical reactions and develop new strategies for optimizing reaction rates and yields.
We hope this article has provided a comprehensive overview of the two key points of the Arrhenius equation. If you have any questions or comments, please feel free to share them with us.
What is the significance of the Arrhenius equation in chemistry?
+The Arrhenius equation is a fundamental concept in chemistry that describes the temperature dependence of reaction rates. It is widely used to analyze and predict the kinetics of chemical reactions.
What is the relationship between activation energy and pre-exponential factor?
+The activation energy and pre-exponential factor are related through the Arrhenius equation. The equation shows that the rate constant of a reaction is proportional to the pre-exponential factor and exponentially dependent on the activation energy.
How does the Arrhenius equation affect the rate constant of a reaction?
+The Arrhenius equation shows that the rate constant of a reaction increases with increasing pre-exponential factor and decreases with increasing activation energy.