Sulfur Electron Configuration: Unlocking the Secrets of the Atom

Understanding the electron configuration of sulfur is crucial for grasping various chemical and physical properties of this element. Electron configuration refers to the arrangement of electrons in an atom, and it plays a significant role in determining the element's reactivity, atomic radius, and other characteristics. In this article, we will delve into the world of sulfur electron configuration, exploring its importance, the step-by-step process of determining it, and the practical applications of this knowledge.
Why is Electron Configuration Important?
Electron configuration is vital for understanding the behavior of atoms and molecules. It helps chemists and physicists predict how elements will react with each other, what types of bonds they will form, and what properties they will exhibit. By knowing the electron configuration of an element, scientists can also determine its position in the periodic table, which is essential for understanding the relationships between elements and their properties.
The 6-Step Guide to Determining Sulfur Electron Configuration

Determining the electron configuration of sulfur involves a step-by-step process that requires an understanding of atomic physics and chemistry. Here are the six steps to follow:
Step 1: Determine the Atomic Number of Sulfur
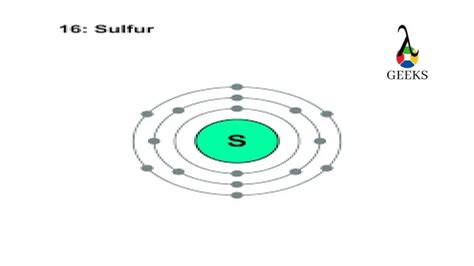
The atomic number of an element is the number of protons present in its atomic nucleus. Sulfur has an atomic number of 16, which means it has 16 protons in its nucleus.
Step 2: Determine the Number of Electrons in a Neutral Sulfur Atom
A neutral atom has an equal number of protons and electrons. Since sulfur has 16 protons, it also has 16 electrons.
Step 3: Fill the Electron Shells
Electron shells are the energy levels or orbitals that electrons occupy around the nucleus. The electron shells are filled in a specific order, following the Aufbau principle and the Pauli Exclusion Principle. The order of filling is: 1s, 2s, 2p, 3s, 3p, 4s, 3d, etc.
Step 4: Determine the Electron Configuration of Sulfur
Using the steps above, we can determine the electron configuration of sulfur. The first two electrons fill the 1s orbital, the next two fill the 2s orbital, and the next six fill the 2p orbitals. The next two electrons fill the 3s orbital, and the next six fill the 3p orbitals. Therefore, the electron configuration of sulfur is: 1s² 2s² 2p⁶ 3s² 3p⁴.
Step 5: Determine the Valence Electrons
Valence electrons are the electrons in the outermost energy level of an atom, which participate in chemical bonding. Sulfur has six valence electrons, which are the electrons in the 3s and 3p orbitals.
Step 6: Write the Electron Configuration in a Abbreviated Form
The electron configuration can be written in a abbreviated form by using the noble gas core. The noble gas core is the electron configuration of the noble gas that precedes the element in the periodic table. For sulfur, the noble gas core is neon (Ne). Therefore, the electron configuration of sulfur can be written as: [Ne] 3s² 3p⁴.
Practical Applications of Sulfur Electron Configuration

Understanding the electron configuration of sulfur has numerous practical applications in various fields, including:
- Chemistry: Knowing the electron configuration of sulfur helps chemists predict its reactivity and the types of bonds it will form with other elements.
- Materials Science: Sulfur is used in the production of various materials, such as matches, gunpowder, and fertilizers. Understanding its electron configuration helps scientists design new materials with specific properties.
- Environmental Science: Sulfur is a key component of acid rain, and understanding its electron configuration helps scientists develop strategies for reducing its impact on the environment.
Conclusion
In conclusion, understanding the electron configuration of sulfur is crucial for grasping its chemical and physical properties. By following the six-step guide outlined above, scientists can determine the electron configuration of sulfur and use this knowledge to predict its reactivity, atomic radius, and other characteristics. The practical applications of sulfur electron configuration are diverse, ranging from chemistry and materials science to environmental science.
Call to Action

We hope this article has provided you with a comprehensive understanding of sulfur electron configuration. If you have any questions or comments, please feel free to share them with us. We would love to hear from you!
What is the atomic number of sulfur?
+The atomic number of sulfur is 16.
What is the electron configuration of sulfur?
+The electron configuration of sulfur is 1s² 2s² 2p⁶ 3s² 3p⁴.
What are the practical applications of sulfur electron configuration?
+The practical applications of sulfur electron configuration include chemistry, materials science, and environmental science.