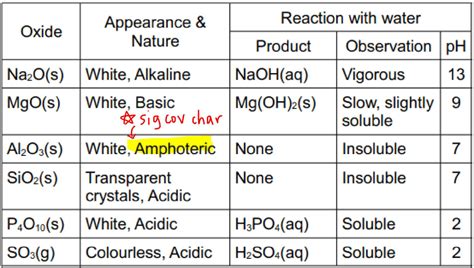
Sodium oxide is a highly reactive compound that plays a significant role in various industrial and chemical processes. When it comes into contact with water, it exhibits distinct and fascinating behavior, leading to the formation of different products. The reaction between sodium oxide and water is a fundamental concept in chemistry, and understanding its various aspects is essential for scientists, researchers, and students alike.
Sodium oxide, also known as sodium monoxide, is a white or yellowish solid with the chemical formula Na2O. It is highly hygroscopic, meaning it readily absorbs moisture from the air, and it reacts vigorously with water to produce sodium hydroxide (NaOH). This reaction is often used in the production of sodium hydroxide, which is a critical component in the manufacture of paper, textiles, and soap.

Exothermic Reaction
One of the most notable aspects of the reaction between sodium oxide and water is its exothermic nature. When sodium oxide is added to water, it rapidly releases heat, causing the mixture to become warm or even hot. This heat release is a result of the strong bond formation between the sodium oxide molecules and the water molecules, leading to the production of sodium hydroxide.
The exothermic reaction between sodium oxide and water can be represented by the following equation:
Na2O (s) + H2O (l) → 2NaOH (aq)
In this equation, sodium oxide (Na2O) reacts with water (H2O) to form sodium hydroxide (NaOH). The "s" and "l" denote the solid and liquid states, respectively, while "aq" indicates an aqueous solution.
Formation of Sodium Hydroxide
As mentioned earlier, the reaction between sodium oxide and water produces sodium hydroxide, a strong base with numerous industrial applications. Sodium hydroxide is a critical component in the manufacture of various products, including paper, textiles, soap, and detergents.
The production of sodium hydroxide through the reaction between sodium oxide and water is a cost-effective and efficient method. The resulting sodium hydroxide solution is highly concentrated, making it ideal for various industrial applications.
Reactions with Acids
Sodium oxide also reacts with acids to produce salts and water. This reaction is known as neutralization, where the sodium oxide reacts with the acid to form a salt and water.
For example, when sodium oxide reacts with hydrochloric acid (HCl), it produces sodium chloride (NaCl) and water:
Na2O (s) + 2HCl (aq) → 2NaCl (aq) + H2O (l)
In this equation, sodium oxide reacts with hydrochloric acid to form sodium chloride and water. This reaction is a classic example of an acid-base reaction, where the base (sodium oxide) reacts with the acid (hydrochloric acid) to form a salt and water.

Formation of Sodium Peroxide
When sodium oxide reacts with oxygen, it forms sodium peroxide (Na2O2). This reaction is highly exothermic and produces a bright yellow solid.
2Na2O (s) + O2 (g) → 2Na2O2 (s)
In this equation, sodium oxide reacts with oxygen to form sodium peroxide. The "g" denotes the gaseous state.
Sodium peroxide is a strong oxidizing agent and is used in various industrial applications, including the production of detergents and the treatment of wastewater.
Conclusion
In conclusion, the reaction between sodium oxide and water is a complex and fascinating process that produces various products, including sodium hydroxide, sodium chloride, and sodium peroxide. The exothermic nature of the reaction makes it highly efficient and cost-effective for industrial applications.
The formation of sodium hydroxide through the reaction between sodium oxide and water is a critical process in the manufacture of various products, including paper, textiles, and soap. The reaction between sodium oxide and acids produces salts and water, making it a fundamental concept in acid-base chemistry.
The reaction between sodium oxide and oxygen produces sodium peroxide, a strong oxidizing agent with various industrial applications.
We hope this article has provided you with a comprehensive understanding of the reactions between sodium oxide and water. If you have any questions or comments, please feel free to share them with us.
FAQ Section
What is the chemical formula for sodium oxide?
+The chemical formula for sodium oxide is Na2O.
What is the product of the reaction between sodium oxide and water?
+The product of the reaction between sodium oxide and water is sodium hydroxide (NaOH).
What is the exothermic reaction between sodium oxide and water used for?
+The exothermic reaction between sodium oxide and water is used for the production of sodium hydroxide, which is a critical component in the manufacture of various products, including paper, textiles, and soap.