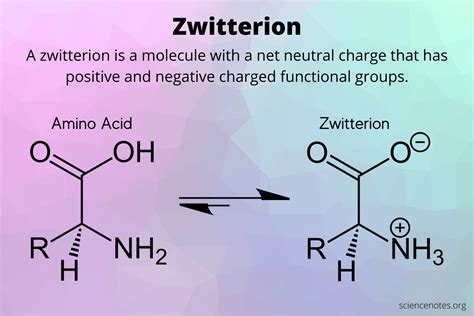
As we continue to explore the intricacies of molecular structures, one fascinating aspect that has garnered significant attention is the zwitterion form of methionine. This naturally occurring amino acid plays a crucial role in various biological processes, and understanding its zwitterion form can provide valuable insights into its behavior and interactions. In this article, we will delve into the world of methionine's zwitterion form, exploring its definition, significance, and the molecular modifications that lead to its formation.

Understanding Zwitterions
To grasp the concept of methionine's zwitterion form, it's essential to understand what zwitterions are. Zwitterions are molecules that contain both a positively charged group and a negatively charged group within the same molecule. This unique characteristic allows zwitterions to exhibit distinct properties, such as increased solubility in water and altered reactivity. In the context of amino acids, zwitterions play a crucial role in protein structure and function.
The Importance of Methionine's Zwitterion Form
Methionine is an essential amino acid, meaning it cannot be synthesized by the human body and must be obtained through dietary sources. Its zwitterion form is particularly significant, as it influences various aspects of methionine's behavior, including:
- Protein structure: Methionine's zwitterion form can affect protein folding and stability, which is critical for proper protein function.
- Enzyme interactions: The zwitterion form of methionine can modulate its interactions with enzymes, influencing catalytic activity and substrate specificity.
- Cellular transport: Methionine's zwitterion form can impact its transport across cell membranes, affecting its availability for various cellular processes.
The Molecular Modifications Leading to Methionine's Zwitterion Form
So, how does methionine adopt its zwitterion form? The process involves a series of molecular modifications that alter the amino acid's charge distribution.

- Deprotonation: The first step involves the loss of a proton (H+ ion) from the amino group, resulting in a negatively charged carboxylate group.
- Protonation: Simultaneously, the carboxyl group gains a proton, becoming a positively charged ammonium group.
- Tautomerization: The resulting molecule undergoes a tautomerization reaction, where the negatively charged carboxylate group migrates to the adjacent carbon atom.
These molecular modifications lead to the formation of methionine's zwitterion form, which is characterized by the presence of both a positively charged ammonium group and a negatively charged carboxylate group.
Practical Applications of Methionine's Zwitterion Form
Understanding methionine's zwitterion form has numerous practical applications in various fields, including:
- Protein engineering: Knowledge of methionine's zwitterion form can inform the design of proteins with specific properties, such as improved stability or catalytic activity.
- Drug development: The zwitterion form of methionine can influence the binding of drugs to proteins, affecting their efficacy and specificity.
- Nutrition and health: A deeper understanding of methionine's zwitterion form can provide insights into its role in human health and disease, informing the development of nutritional supplements and therapeutic strategies.
Conclusion and Future Directions
In conclusion, methionine's zwitterion form is a fascinating aspect of molecular biology, with significant implications for our understanding of protein structure and function. Further research into the molecular modifications leading to this form will continue to reveal the intricacies of methionine's behavior and interactions.

We invite you to share your thoughts and questions about methionine's zwitterion form in the comments below. How do you think this knowledge can be applied in various fields, and what future research directions do you envision?
What is the significance of methionine's zwitterion form in protein structure and function?
+Methionine's zwitterion form can affect protein folding and stability, as well as modulate its interactions with enzymes and influence catalytic activity.
How does the zwitterion form of methionine influence its interactions with drugs?
+The zwitterion form of methionine can affect the binding of drugs to proteins, influencing their efficacy and specificity.
What are some potential applications of understanding methionine's zwitterion form in nutrition and health?
+A deeper understanding of methionine's zwitterion form can provide insights into its role in human health and disease, informing the development of nutritional supplements and therapeutic strategies.