Understanding the Importance of Lysine

Lysine, an essential amino acid, plays a vital role in various bodily functions, including protein synthesis, hormone production, and immune function. Its unique chemical structure allows it to participate in numerous biological processes, making it a crucial component of a balanced diet. However, the behavior of lysine can change significantly depending on the surrounding environment, particularly pH levels.
Lysine's Structure and Properties

At physiological pH (around 7.4), lysine exists in a specific form that enables it to interact with other molecules and perform its biological functions. Understanding the structure and properties of lysine is essential to grasp its behavior at different pH levels.
Lysine's chemical formula is C6H14N2O2, and it contains a positively charged amino group (-NH3+) and a negatively charged carboxyl group (-COO-). This zwitterionic nature allows lysine to participate in various biochemical reactions.
Lysine's Ionizable Groups
Lysine has three ionizable groups:
- The alpha-amino group (-NH3+)
- The alpha-carboxyl group (-COO-)
- The epsilon-amino group (-NH3+) on the side chain
These groups can accept or donate protons, depending on the pH, influencing lysine's overall charge and behavior.
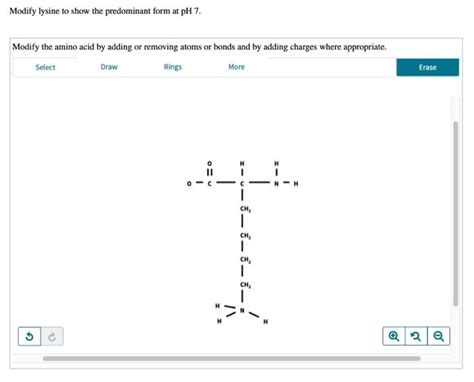
pH 7: The Predominant Form of Lysine

At pH 7, the predominant form of lysine is the zwitterion, with both the alpha-amino and alpha-carboxyl groups ionized. This form is crucial for lysine's biological functions, as it allows the molecule to interact with other charged particles, such as ions, proteins, and membranes.
The pKa values of lysine's ionizable groups determine the pH at which they are ionized or protonated. At pH 7, the alpha-amino group has a pKa of around 9.0, while the alpha-carboxyl group has a pKa of approximately 2.2. The epsilon-amino group on the side chain has a pKa of around 10.5.
Implications of Lysine's Predominant Form at pH 7
The zwitterionic form of lysine at pH 7 has significant implications for its biological functions:
- Protein synthesis: Lysine's zwitterionic form allows it to participate in protein synthesis, as it can interact with other amino acids and the ribosome.
- Hormone regulation: Lysine's charge and structure enable it to bind to hormone receptors, influencing hormone production and activity.
- Immune function: Lysine's zwitterionic form facilitates its interaction with immune cells, such as T-cells and macrophages, supporting immune function.
Conclusion: The Significance of Lysine's Predominant Form

In conclusion, understanding the predominant form of lysine at pH 7 is crucial for grasping its biological functions and importance. The zwitterionic form of lysine at pH 7 enables it to participate in various biochemical processes, including protein synthesis, hormone regulation, and immune function. Further research on lysine's behavior at different pH levels can provide valuable insights into its biological roles and potential applications.
Call to Action
We invite you to share your thoughts on the importance of lysine's predominant form at pH 7. How do you think understanding lysine's behavior at different pH levels can impact our knowledge of its biological functions? Share your comments below!
What is the chemical formula of lysine?
+The chemical formula of lysine is C6H14N2O2.
What is the pKa value of lysine's alpha-amino group?
+The pKa value of lysine's alpha-amino group is around 9.0.
What is the biological significance of lysine's zwitterionic form at pH 7?
+Lysine's zwitterionic form at pH 7 enables it to participate in protein synthesis, hormone regulation, and immune function.