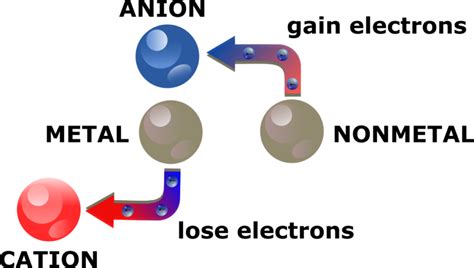
The world of chemistry is fascinating, and one of the fundamental concepts that underlie many chemical reactions is the formation of ions. Ions are atoms or molecules that have gained or lost electrons, resulting in a net positive or negative charge. In this article, we will delve into the world of metals and explore the three ways they lose electrons to form ions.

Metals are a class of elements that are typically shiny, malleable, and good conductors of electricity. They are also known for their ability to lose electrons easily, which makes them prone to forming ions. The process of losing electrons to form ions is known as ionization.
What is Ionization?
Ionization is the process by which an atom or molecule gains or loses electrons to form an ion. In the case of metals, ionization typically involves the loss of one or more electrons to form a positively charged ion. This process can occur through various means, including chemical reactions, heat, or light.
Importance of Ionization
Ionization is an important concept in chemistry, as it plays a crucial role in many chemical reactions. Ions are highly reactive, and their formation can lead to the creation of new compounds and the breakdown of existing ones. Understanding how metals lose electrons to form ions is essential for understanding many chemical processes, including corrosion, electrochemistry, and the behavior of ionic compounds.
3 Ways Metals Lose Electrons to Form Ions
Metals can lose electrons to form ions through various mechanisms. Here are three common ways metals lose electrons to form ions:
1. Electrolysis
Electrolysis is a process in which an electric current is used to drive a chemical reaction. In the case of metals, electrolysis can be used to remove electrons from the metal atom, resulting in the formation of a positively charged ion. This process is commonly used in electroplating, where a metal is deposited onto a surface using an electric current.

2. Chemical Reactions
Metals can also lose electrons to form ions through chemical reactions. For example, when a metal reacts with an acid, the metal can lose electrons to form a positively charged ion. This process is known as oxidation, and it is a common mechanism for the formation of ions in chemical reactions.
3. Heat
Heat can also be used to remove electrons from metal atoms, resulting in the formation of ions. This process is known as thermal ionization, and it occurs when a metal is heated to a high temperature. The heat energy excites the electrons in the metal, causing them to be ejected from the atom and resulting in the formation of a positively charged ion.

Examples of Metals Losing Electrons to Form Ions
Here are some examples of metals losing electrons to form ions:
- Sodium (Na) loses an electron to form a positively charged ion (Na+)
- Calcium (Ca) loses two electrons to form a positively charged ion (Ca2+)
- Aluminum (Al) loses three electrons to form a positively charged ion (Al3+)

Conclusion
In conclusion, metals can lose electrons to form ions through various mechanisms, including electrolysis, chemical reactions, and heat. Understanding how metals lose electrons to form ions is essential for understanding many chemical processes, including corrosion, electrochemistry, and the behavior of ionic compounds. We hope this article has provided you with a deeper understanding of the world of metals and ions.
Now that you've read this article, we'd love to hear from you! What are some other ways metals can lose electrons to form ions? Share your thoughts in the comments below!
What is the difference between a metal and an ion?
+A metal is an atom or molecule that has not gained or lost electrons, while an ion is an atom or molecule that has gained or lost electrons, resulting in a net positive or negative charge.
What is electrolysis?
+Electrolysis is a process in which an electric current is used to drive a chemical reaction, resulting in the formation of ions.
What is thermal ionization?
+Thermal ionization is the process by which heat is used to remove electrons from an atom or molecule, resulting in the formation of ions.