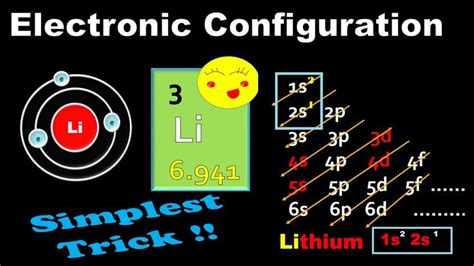
Lithium is the third element in the periodic table, with an atomic number of 3 and an atomic mass of 6.94 u (unified atomic mass units). It is a soft, silvery-white alkali metal that is highly reactive and has a number of unique properties. In this article, we will delve into the world of electron configuration and explore the long form of lithium's electron configuration.
Lithium Electron Configuration: Understanding the Basics

Before we dive into the long form of lithium's electron configuration, it's essential to understand the basics of electron configuration. Electron configuration is a way of describing the arrangement of electrons in an atom. It is a fundamental concept in chemistry and physics, as it helps us understand the behavior of elements and their interactions with other elements.
The electron configuration of an atom is typically written in a shorthand notation, which shows the energy level and orbital type of each electron. The energy levels are represented by numbers (1, 2, 3, etc.), and the orbital types are represented by letters (s, p, d, f, etc.). The number of electrons in each energy level is indicated by a superscript number.
Understanding the Energy Levels and Orbitals
To understand the electron configuration of lithium, we need to understand the energy levels and orbitals involved. The energy levels are:
- 1s (first energy level, s-orbital)
- 2s (second energy level, s-orbital)
- 2p (second energy level, p-orbital)
The s-orbitals are spherical in shape and can hold a maximum of two electrons. The p-orbitals are dumbbell-shaped and can hold a maximum of six electrons.
Lithium Electron Configuration Long Form

Now that we have a basic understanding of electron configuration and the energy levels and orbitals involved, let's take a look at the long form of lithium's electron configuration:
1s² 2s¹
This configuration indicates that the first energy level (1s) is fully occupied with two electrons, and the second energy level (2s) has one electron. The superscript numbers indicate the number of electrons in each energy level.
Understanding the Electron Configuration Notation
The notation used to write the electron configuration of lithium is:
- The number (1, 2, 3, etc.) represents the energy level.
- The letter (s, p, d, f, etc.) represents the orbital type.
- The superscript number indicates the number of electrons in the energy level.
For example, the notation "1s²" indicates that the first energy level (1s) has two electrons.
Benefits of Understanding Lithium's Electron Configuration

Understanding the electron configuration of lithium has several benefits:
- Predicting Chemical Properties: The electron configuration of an element can help predict its chemical properties, such as its reactivity and the types of compounds it can form.
- Understanding Chemical Bonding: The electron configuration of an element can help us understand how it forms chemical bonds with other elements.
- Identifying Isotopes: The electron configuration of an element can help us identify its isotopes and understand their properties.
Real-World Applications of Lithium's Electron Configuration
Lithium's electron configuration has several real-world applications:
- Batteries: Lithium's electron configuration makes it an ideal element for use in batteries, particularly lithium-ion batteries.
- Pharmaceuticals: Lithium is used in the production of certain pharmaceuticals, such as lithium carbonate, which is used to treat bipolar disorder.
- Nuclear Applications: Lithium is used in the production of nuclear fuels and in the development of nuclear reactors.
Conclusion: Unlocking the Secrets of Lithium's Electron Configuration

In conclusion, the electron configuration of lithium is a fundamental concept in chemistry and physics that helps us understand the behavior of this element and its interactions with other elements. By understanding the long form of lithium's electron configuration, we can unlock the secrets of its chemical properties and identify its real-world applications.
Take the Next Step: Share Your Thoughts and Questions!
We hope this article has provided you with a comprehensive understanding of lithium's electron configuration. If you have any questions or comments, please share them with us in the comments section below. Let's keep the conversation going!
What is the atomic number of lithium?
+The atomic number of lithium is 3.
What is the electron configuration of lithium?
+The electron configuration of lithium is 1s² 2s¹.
What are the real-world applications of lithium's electron configuration?
+Lithium's electron configuration has several real-world applications, including batteries, pharmaceuticals, and nuclear applications.