The periodic table is a powerful tool that helps us understand the properties and behavior of elements. One crucial aspect of an element's identity is its electron configuration, which describes how electrons are arranged in an atom. In this article, we will explore the steps to write the Krypton electron configuration, a noble gas with the atomic number 36.
The Importance of Electron Configuration
Electron configuration is a fundamental concept in chemistry that helps us predict an element's chemical properties, reactivity, and behavior. It's essential to understand how electrons are arranged in an atom to comprehend various chemical reactions, bonding, and the formation of compounds. By mastering electron configuration, you'll gain a deeper understanding of the periodic table and the relationships between elements.
What is Krypton?
Krypton is a noble gas with the atomic number 36 and the symbol Kr. It's a colorless, odorless, and tasteless gas that is chemically inert, meaning it doesn't react with other elements easily. Krypton is used in various applications, including lighting, lasers, and magnetic resonance imaging (MRI) machines.
Step 1: Determine the Atomic Number of Krypton
The atomic number of an element is the number of protons present in its atomic nucleus. Krypton's atomic number is 36, which means it has 36 protons in its nucleus. This information is crucial for writing the electron configuration.

Step 2: Identify the Number of Energy Levels (Shells)
The energy levels or shells of an atom are the regions around the nucleus where electrons are found. The number of energy levels in an atom is determined by the atomic number. For Krypton, with an atomic number of 36, there are four energy levels or shells.
Understanding Energy Levels (Shells)
Energy levels or shells are designated by integers (1, 2, 3, etc.), starting from the nucleus. Each energy level has a specific capacity for electrons, which is determined by the formula 2n^2, where n is the energy level number. The first energy level can hold up to 2 electrons, the second energy level can hold up to 8 electrons, and so on.
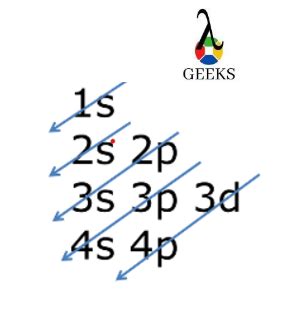
Step 3: Fill in the Electrons According to the Aufbau Principle
The Aufbau principle states that electrons occupy the lowest available energy levels in an atom. To write the electron configuration of Krypton, we'll fill in the electrons according to this principle.

Starting from the first energy level, we'll fill in the electrons:
- Energy level 1: 2 electrons (1s^2)
- Energy level 2: 8 electrons (2s^2 2p^6)
- Energy level 3: 18 electrons (3s^2 3p^6 3d^10)
- Energy level 4: 8 electrons (4s^2 4p^6)
Step 4: Write the Electron Configuration in the Correct Format
Now that we've filled in the electrons according to the Aufbau principle, we can write the electron configuration in the correct format:
1s^2 2s^2 2p^6 3s^2 3p^6 3d^10 4s^2 4p^6
This is the electron configuration of Krypton.
Step 5: Verify the Electron Configuration
To verify the electron configuration, we can check if the total number of electrons matches the atomic number of Krypton. In this case, the total number of electrons is 36, which matches the atomic number.

Tips and Tricks
- Always start with the Aufbau principle when writing electron configurations.
- Use the periodic table to identify the atomic number and energy levels of an element.
- Verify the electron configuration by checking if the total number of electrons matches the atomic number.
Conclusion
Writing the electron configuration of Krypton requires understanding the atomic number, energy levels, and the Aufbau principle. By following these steps, you'll be able to write the electron configuration of Krypton and other elements with confidence. Remember to verify your work to ensure accuracy.
We hope this article has helped you understand the steps to write the Krypton electron configuration. If you have any questions or comments, please share them below.
What is the atomic number of Krypton?
+The atomic number of Krypton is 36.
What is the Aufbau principle?
+The Aufbau principle states that electrons occupy the lowest available energy levels in an atom.
How do I verify the electron configuration?
+Verify the electron configuration by checking if the total number of electrons matches the atomic number.